miRNA-6715-5p Inhibits Cellular Proliferation and Invasion in Colorectal Cancer by Directly Targeting CST4
- PMID: 34194498
- PMCID: PMC8181091
- DOI: 10.1155/2021/7615712
miRNA-6715-5p Inhibits Cellular Proliferation and Invasion in Colorectal Cancer by Directly Targeting CST4
Abstract
Background: Data on the correlation between CST4 and colorectal cancer (CRC) metastasis are scarce. The aim of this study was to analyze CST4 expression and investigate its biological roles and related microRNA- (miRNA-) mediated regulation in CRC.
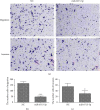
Methods: The expression of CST4 was examined in cancer tissues and their corresponding adjacent normal tissues from 40 gastric adenocarcinoma patients. The expression level of CST4 in specimens (cancer and normal tissues) was assessed through immunohistochemistry and/or quantitative polymerase chain reaction. miRNAs targeting CST4 in CRC were predicted by bioinformatics software. CST4 was knocked down in HCT116 cells and candidate miRNAs were transfected into HCT116 cells, and the effects of CST4 knockdown and miRNA transfection on cell proliferation and invasion were examined using CCK8, cell colony formation, and Transwell migration assays. Luciferase double-reporter assays were performed to verify the relationship between miRNA and CST4.
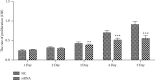
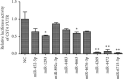
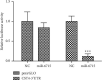
Results: The expression of CST4 in CRC tissues was significantly higher than that in normal paracancerous tissues, but the results for miRNA-6715-5p were opposite. Regardless of CST4 knockdown or miRNA-6715-5p overexpression, the proliferation and invasion ability of HCT116 cells decreased significantly. Luciferase double-reporter assays showed that the upregulation of miR-6715-5p significantly reduced the luciferase activities of the CST4 3'-UTR plasmid in HCT116 cells.
Conclusion: CST4 may be involved in CRC proliferation and metastasis. miRNA-6715-5p directly targets CST4 and negatively regulates its expression.
Copyright © 2021 Ding Shi et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures











Similar articles
-
MiR-335-5p Inhibits Cell Proliferation, Migration and Invasion in Colorectal Cancer through Downregulating LDHB.J BUON. 2019 May-Jun;24(3):1128-1136. J BUON. 2019. PMID: 31424671
-
miR-17-5p promotes the invasion and migration of colorectal cancer by regulating HSPB2.J Cancer. 2022 Jan 1;13(3):918-931. doi: 10.7150/jca.65614. eCollection 2022. J Cancer. 2022. PMID: 35154459 Free PMC article.
-
AKAP12 Endogenous Transcripts Suppress The Proliferation, Migration And Invasion Of Colorectal Cancer Cells By Directly Targeting oncomiR-183-5p.Onco Targets Ther. 2019 Oct 8;12:8301-8310. doi: 10.2147/OTT.S207600. eCollection 2019. Onco Targets Ther. 2019. PMID: 31632079 Free PMC article.
-
Integrated analysis identifies microRNA-195 as a suppressor of Hippo-YAP pathway in colorectal cancer.J Hematol Oncol. 2017 Mar 29;10(1):79. doi: 10.1186/s13045-017-0445-8. J Hematol Oncol. 2017. PMID: 28356122 Free PMC article.
-
[miRNA-96-5p inhibits the proliferation and migration of gastric cancer cells by targeting FoxQ1].Zhonghua Zhong Liu Za Zhi. 2019 Mar 23;41(3):193-199. doi: 10.3760/cma.j.issn.0253-3766.2019.03.008. Zhonghua Zhong Liu Za Zhi. 2019. PMID: 30917455 Chinese.
Cited by
-
Investigating the prognostic and predictive value of the type II cystatin genes in gastric cancer.BMC Cancer. 2023 Nov 17;23(1):1122. doi: 10.1186/s12885-023-11550-6. BMC Cancer. 2023. PMID: 37978366 Free PMC article.
-
Type 2 Cystatins and Their Roles in the Regulation of Human Immune Response and Cancer Progression.Cancers (Basel). 2023 Nov 10;15(22):5363. doi: 10.3390/cancers15225363. Cancers (Basel). 2023. PMID: 38001623 Free PMC article. Review.
-
Downregulated miRNA-491-3p accelerates colorectal cancer growth by increasing uMtCK expression.PeerJ. 2022 Nov 28;10:e14285. doi: 10.7717/peerj.14285. eCollection 2022. PeerJ. 2022. PMID: 36518289 Free PMC article.
References
-
- Yoneda K., Iida H., Endo H., et al. Identification of cystatin SN as a novel tumor marker for colorectal cancer. International Journal of Oncology. 2009;35:33–40. - PubMed
LinkOut - more resources
Full Text Sources

