A platform for locoregional T-cell immunotherapy to control HNSCC recurrence following tumor resection
- PMID: 34194619
- PMCID: PMC8238246
- DOI: 10.18632/oncotarget.27982
A platform for locoregional T-cell immunotherapy to control HNSCC recurrence following tumor resection
Abstract
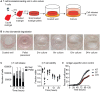
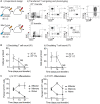
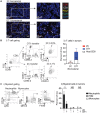
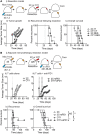
Surgical resection of head and neck squamous-cell carcinoma (HNSCC) is associated with high rates of local and distant recurrence, partially mitigated by adjuvant therapy. A pre-existing immune response in the patient's tumor is associated with better outcomes following treatment with conventional therapies, but improved options are needed for patients with poor anti-tumor immunity. We hypothesized that local delivery of tumor antigen-specific T-cells into the resection cavity following surgery would direct T-cells to residual antigens in the margins and draining lymphatics and present a platform for T-cell-targeted immunotherapy. We loaded T-cells into a biomaterial that conformed to the resection cavity and demonstrated that it could release T-cells that retained their functional activity in-vitro, and in a HNSCC model in-vivo. Locally delivered T-cells loaded in a biomaterial were equivalent in control of established tumors to intravenous adoptive T-cell transfer, and resulted in the systemic circulation of tumor antigen-specific T-cells as well as local accumulation in the tumor. We demonstrate that adjuvant therapy with anti-PD1 following surgical resection was ineffective unless combined with local delivery of T-cells. These data demonstrate that local delivery of tumor-specific T-cells is an efficient option to convert tumors that are unresponsive to checkpoint inhibitors to permit tumor cures.
Keywords: T-cell; biomaterial; head and neck cancer; immunotherapy; intratumoral.
Copyright: © 2021 Sharon et al.
Conflict of interest statement
CONFLICTS OF INTEREST MJG and MRC receive research funding from Bristol Myers-Squibb, Jounce, and Mavupharma that is unrelated to the content of this manuscript. The remaining authors declare no competing interests. Funders had no input in the content of the manuscript.
Figures

References
-
- Jones AS, Bin Hanafi Z, Nadapalan V, Roland NJ, Kinsella A, Helliwell TR. Do positive resection margins after ablative surgery for head and neck cancer adversely affect prognosis? A study of 352 patients with recurrent carcinoma following radiotherapy treated by salvage surgery. Br J Cancer. 1996; 74:128–32. 10.1038/bjc.1996.327. - DOI - PMC - PubMed
-
- Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, Ozsahin EM, Jacobs JR, Jassem J, Ang KK, Lefèbvre JL. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 2005; 27:843–50. 10.1002/hed.20279. - DOI - PubMed
-
- Friedman J, Moore EC, Zolkind P, Robbins Y, Clavijo PE, Sun L, Greene S, Morisada MV, Mydlarz WK, Schmitt N, Hodge JW, Schreiber H, Van Waes C, et al.. Neoadjuvant PD-1 Immune Checkpoint Blockade Reverses Functional Immunodominance among Tumor Antigen-Specific T Cells. Clin Cancer Res. 2020; 26:679–89. 10.1158/1078-0432.CCR-19-2209. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

