Synthetic Formatotrophs for One-Carbon Biorefinery
- PMID: 34194943
- PMCID: PMC8224422
- DOI: 10.1002/advs.202100199
Synthetic Formatotrophs for One-Carbon Biorefinery
Abstract
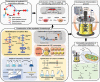
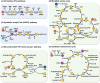
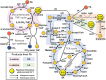
The use of CO2 as a carbon source in biorefinery is of great interest, but the low solubility of CO2 in water and the lack of efficient CO2 assimilation pathways are challenges to overcome. Formic acid (FA), which can be easily produced from CO2 and more conveniently stored and transported than CO2, is an attractive CO2-equivalent carbon source as it can be assimilated more efficiently than CO2 by microorganisms and also provides reducing power. Although there are native formatotrophs, they grow slowly and are difficult to metabolically engineer due to the lack of genetic manipulation tools. Thus, much effort is exerted to develop efficient FA assimilation pathways and synthetic microorganisms capable of growing solely on FA (and CO2). Several innovative strategies are suggested to develop synthetic formatotrophs through rational metabolic engineering involving new enzymes and reconstructed FA assimilation pathways, and/or adaptive laboratory evolution (ALE). In this paper, recent advances in development of synthetic formatotrophs are reviewed, focusing on biological FA and CO2 utilization pathways, enzymes involved and newly developed, and metabolic engineering and ALE strategies employed. Also, future challenges in cultivating formatotrophs to higher cell densities and producing chemicals from FA and CO2 are discussed.
Keywords: formatotroph; formic acid assimilation; one‐carbon biorefinery; systems metabolic engineering.
© 2021 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kumar A., Ergas S., Yuan X., Sahu A., Zhang Q., Dewulf J., Malcata F. X., Langenhove H., Trends. Biotechnol. 2010, 28, 371. - PubMed
-
- Irfan M., Bai Y., Zhou L., Kazmi M., Yuan S., Mbadinga S. M., Yang S. Z., Sand W., Gu J. D., Mu B. Z., Bioresour. Technol. 2019, 288, 121401. - PubMed
-
- Thomas D. M., Mechery J., Paulose V., Environ. Sci. Pollut. Res. 2016, 23, 16926. - PubMed
-
- Yuan M., Kummer M. J., Minteer S. D., Chem. ‐ Eur. J. 2019, 25, 14258. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
