A Single Dose of Nitrate Increases Resilience Against Acidification Derived From Sugar Fermentation by the Oral Microbiome
- PMID: 34195102
- PMCID: PMC8238012
- DOI: 10.3389/fcimb.2021.692883
A Single Dose of Nitrate Increases Resilience Against Acidification Derived From Sugar Fermentation by the Oral Microbiome
Abstract
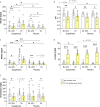
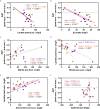
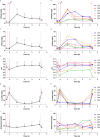
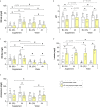
Tooth decay starts with enamel demineralization due to an acidic pH, which arises from sugar fermentation by acidogenic oral bacteria. Previous in vitro work has demonstrated that nitrate limits acidification when incubating complex oral communities with sugar for short periods (e.g., 1-5 h), driven by changes in the microbiota metabolism and/or composition. To test whether a single dose of nitrate can reduce acidification derived from sugar fermentation in vivo, 12 individuals received a nitrate-rich beetroot supplement, which was compared to a placebo in a blinded crossover setting. Sucrose-rinses were performed at baseline and 2 h after supplement or placebo intake, and the salivary pH, nitrate, nitrite, ammonium and lactate were measured. After nitrate supplement intake, the sucrose-induced salivary pH drop was attenuated when compared with the placebo (p < 0.05). Salivary nitrate negatively correlated with lactate production and positively with ΔpH after sucrose exposure (r= -0.508 and 0.436, respectively, both p < 0.05). Two additional pilot studies were performed to test the effect of sucrose rinses 1 h (n = 6) and 4 h (n = 6) after nitrate supplement intake. In the 4 h study, nitrate intake was compared with water intake and bacterial profiles were analysed using 16S rRNA gene Illumina sequencing and qPCR detection of Rothia. Sucrose rinses caused a significant pH drop (p < 0.05), except 1 h and 4 h after nitrate supplement intake. After 4 h of nitrate intake, there was less lactate produced compared to water intake (p < 0.05) and one genus; Rothia, increased in abundance. This small but significant increase was confirmed by qPCR (p < 0.05). The relative abundance of Rothia and Neisseria negatively correlated with lactate production (r = -0.601 and -0.669, respectively) and Neisseria positively correlated with pH following sucrose intake (r = 0.669, all p < 0.05). Together, these results show that nitrate can acutely limit acidification when sugars are fermented, which appears to result from lactate usage by nitrate-reducing bacteria. Future studies should assess the longitudinal impact of daily nitrate-rich vegetable or supplement intake on dental health.
Keywords: Rothia; acidification; caries; nitrate; oral microbiota; pH buffering capacity; resilience; saliva.
Copyright © 2021 Rosier, Palazón, García-Esteban, Artacho, Galiana and Mira.
Conflict of interest statement
AM and BR are co-inventors in a pending patent application owned by the FISABIO Institute, which protects the use of nitrate as a prebiotic. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

