Detection of Mycobacterium tuberculosis Complex Bacilli and Nucleic Acids From Tongue Swabs in Young, Hospitalized Children
- PMID: 34195103
- PMCID: PMC8238041
- DOI: 10.3389/fcimb.2021.696379
Detection of Mycobacterium tuberculosis Complex Bacilli and Nucleic Acids From Tongue Swabs in Young, Hospitalized Children
Abstract
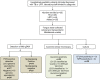
Diagnosis of tuberculosis in pediatric patients remains challenging due to inherent difficulties associated with obtaining respiratory samples for molecular and culture-based testing. To address this, recent studies have highlighted the utility of tongue swabs to detect Mycobacterium tuberculosis genomic DNA in the oral epithelia of tuberculosis infected adults. It is unknown whether tongue swabs have similar utility for diagnosis of childhood tuberculosis and if the presence of DNA in these swabs was associated with whole bacilli. We therefore sought to conduct a preliminary assessment of the utility of tongue swabs to detect tubercle bacilli and their associated genetic material in young children. For this, we recruited hospitalized children with clinically diagnosed tuberculosis (n = 26) or lower respiratory tract infection (LRTI, n = 9). These categories were blinded for downstream laboratory tests, which included PCR, spoligotyping, smear microscopy, and culture. Mtb genomic DNA was detected by PCR only in clinically diagnosed TB cases [11/26 (31.4%)] and not in cases with LRTI. Of these, 5/11 [45.5%] were associated with a spoligotype. Spoligotyping also detected an additional six specimens that were negative by PCR. Using smear microscopy, 19/26 [73.1%] and 4/9 [44.4] were Mtb positive in the tuberculosis or LRTI categories respectively. We noted positive results on all three tests in 5/26 [19.2%] in the tuberculosis category and 0/9 in the LRTI category. All specimens were culture negative. Collectively, these preliminary data present a compelling case for broader testing of tongue swabs to diagnose tuberculosis in children where obtaining standard sputum specimens is not easy.
Keywords: auramine staining; pediatric tuberculosis; qPCR; spoligotyping; tongue swabs.
Copyright © 2021 Ealand, Peters, Jacobs, Sewcharran, Ghoor, Golub, Brahmbhatt, Martinson, Dangor, Lala and Kana.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Brosch R. G., Gordon S. V., Eiglmeier K., Garnier T., Tekaia F., Yeramian E., et al. . (2000). “Genomics, Biology, and Evolution of the Mycobacterium Tuberculosis Complex,” in Molecular Genetics of Mycobacteria. Eds. Gf H., Wr J. (Washington DC: American Society for Microbiology Press; ), 19–36.
-
- Brudey K., Driscoll J. R., Rigouts L., Prodinger W. M., Gori A., Al-Hajoj S. A., et al. . (2006). Mycobacterium Tuberculosis Complex Genetic Diversity: Mining the Fourth International Spoligotyping Database (SpolDB4) for Classification, Population Genetics and Epidemiology. BMC Microbiol. 6, 23. 10.1186/1471-2180-6-23 - DOI - PMC - PubMed
-
- Chengalroyen M. D., Beukes G. M., Gordhan B. G., Streicher E. M., Churchyard G., Hafner R., et al. . (2016). Detection and Quantification of Differentially Culturable Tubercle Bacteria in Sputum From Patients With Tuberculosis. Am. J. Respir. Crit. Care Med. 194, 1532–1540. 10.1164/rccm.201604-0769OC - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

