Morphologies and Structure of Brain Lipid Membrane Dispersions
- PMID: 34195192
- PMCID: PMC8236638
- DOI: 10.3389/fcell.2021.675140
Morphologies and Structure of Brain Lipid Membrane Dispersions
Abstract
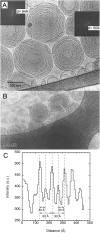
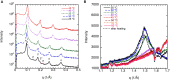
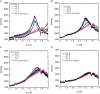
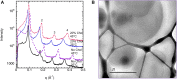
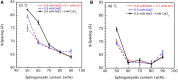
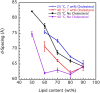
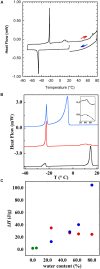
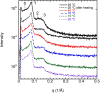
This study aims to explore the variety of previously unknown morphologies that brain lipids form in aqueous solutions. We study how these structures are dependent on cholesterol content, salt solution composition, and temperature. For this purpose, dispersions of porcine sphingomyelin with varying amounts of cholesterol as well as dispersions of porcine brain lipid extracts were investigated. We used cryo-TEM to investigate the dispersions at high-salt solution content together with small-angle (SAXD) and wide-angle X-ray diffraction (WAXD) and differential scanning calorimetry (DSC) for dispersions in the corresponding salt solution at high lipid content. Sphingomyelin forms multilamellar vesicles in large excess of aqueous salt solution. These vesicles appear as double rippled bilayers in the images and as split Bragg peaks in SAXD together with a very distinct lamellar phase pattern. These features disappear with increasing temperature, and addition of cholesterol as the WAXD data shows that the peak corresponding to the chain crystallinity disappears. The dispersions of sphingomyelin at high cholesterol content form large vesicular type of structures with smooth bilayers. The repeat distance of the lamellar phase depends on temperature, salt solution composition, and slightly with cholesterol content. The brain lipid extracts form large multilamellar vesicles often attached to assemblies of higher electron density. We think that this is probably an example of supra self-assembly with a multiple-layered vesicle surrounding an interior cubic microphase. This is challenging to resolve. DSC shows the presence of different kinds of water bound to the lipid aggregates as a function of the lipid content. Comparison with the effect of lithium, sodium, and calcium salts on the structural parameters of the sphingomyelin and the morphologies of brain lipid extract morphologies demonstrate that lithium has remarkable effects also at low content.
Keywords: X-ray diffraction; brain lipid; cholesterol; cryo-TEM; specific ion effects; sphingomyelin; structure and morphology.
Copyright © 2021 Alfredsson, Lo Nostro, Ninham and Nylander.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Agranoff B. W., Benjamins J. A., Hajra A. K. P. (1999). “Properties of brain lipids,” in Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6 Edn, eds Siegel G. J., Agranoff B. W., Albers R. W., Fisher S. K., Uhler M. D. (Philadelphia, PA: Lippincott-Raven; ).
-
- Angelova A., Angelov B., Drechsler M., Bizien T., Gorshkova Y. E., Deng Y. (2021). Plasmalogen-based liquid crystalline multiphase structures involving docosapentaenoyl derivatives inspired by biological cubic membranes. Front. Cell. Dev. Biol. 9:617984. 10.3389/fcell.2021.617984 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

