Preparation of the intact rodent organ of Corti for RNAscope and immunolabeling, confocal microscopy, and quantitative analysis
- PMID: 34195667
- PMCID: PMC8233256
- DOI: 10.1016/j.xpro.2021.100544
Preparation of the intact rodent organ of Corti for RNAscope and immunolabeling, confocal microscopy, and quantitative analysis
Abstract
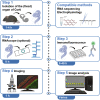
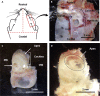
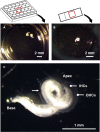
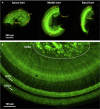
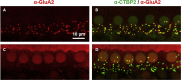
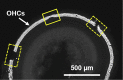
This protocol describes the preparation of the mouse organ of Corti for RNAscope, immunolabeling, confocal microscopy, and quantitative image analysis to examine transcript and protein localization, sensory hair cells, and synapses. This protocol can be applied to mice and other rodents (juvenile and adult) and can be adapted for other techniques, including electrophysiology and RNA sequencing. This protocol features minimal tissue processing to preserve viability for downstream assays, while isolating the organ of Corti is the most challenging step. For additional details on the use and execution of this protocol, please refer to McLean et al. (2009); Schuth et al. (2014); Lingle et al. (2019); Pyott et al. (2020).
Keywords: Antibody; In Situ Hybridization; Microscopy; Molecular/Chemical Probes; Neuroscience.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Feng G., Mellor R.H., Bernstein M., Keller-Peck C., Nguyen Q.T., Wallace M., Nerbonne J.M., Lichtman J.W., Sanes J.R. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. - PubMed
-
- Goutman J.D., Pyott S.J. Whole-Cell Patch-Clamp Recording of Mouse and Rat Inner Hair Cells in the Intact Organ of Corti. Methods Mol. Biol. 2016;1427:471–485. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

