Consensus Guidelines for the Prevention and Management of Periprocedural Complications of Transcatheter Patent Ductus Arteriosus Closure with the Amplatzer Piccolo Occluder in Extremely Low Birth Weight Infants
- PMID: 34195869
- PMCID: PMC8292293
- DOI: 10.1007/s00246-021-02665-3
Consensus Guidelines for the Prevention and Management of Periprocedural Complications of Transcatheter Patent Ductus Arteriosus Closure with the Amplatzer Piccolo Occluder in Extremely Low Birth Weight Infants
Abstract
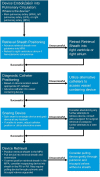
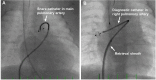
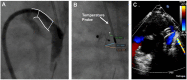
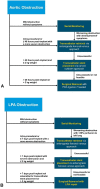
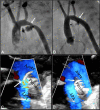
Transcatheter closure of patent ductus arteriosus (PDA) in premature infants is a feasible, safe, and an effective alternative to surgical ligation and may be performed with an implant success rate of 97%. Major procedural complications related to transcatheter PDA closure in extremely low birth weight (ELBW) infants are relatively infrequent (< 3%) ,but may be associated with a fatality if not optimally managed. Operators performing transcatheter PDA closures should be knowledgeable about these potential complications and management options. Prompt recognition and treatment are often necessary to avoid serious consequences. With strict guidelines on operator training, proctoring requirements, and technical refinements, transcatheter PDA closure in ELBW infants can be performed safely with low complication rates. This article summarizes the consensus guidelines put forward by a panel of physicians for the prevention and management of periprocedural complications of transcatheter PDA closure with the Amplatzer Piccolo Occluder in ELBW infants.
Keywords: Amplatzer Piccolo Occluder; Aortic obstruction; Cardiovascular injury; Device embolization; Device migration; Device protrusion; Pulmonary artery obstruction; Transcatheter PDA closure; Tricuspid regurgitation.
© 2021. The Author(s).
Conflict of interest statement
Shyam Sathanandam is a proctor/consultant for Abbott. Dan Gutfinger is a full-time employee of Abbott. Brian Morray is a consultant for Medtronic and proctor for Abbott. Darren Berman is a proctor/consultant for Abbott, Edwards, Medtronic. Matthew Gillespie is a proctor/consultant for Abbott, Medtronic. T. Forbes is a proctor/consultant for Abbott, Edwards, AcuNav/Biosence Webster, B. Braun Medical, Siemens, Medtronic. Jason Johnson is a consultant for Abbott. Ruchira Garg is a consultant for Abbott. Sophie Malekzadeh-Milani is a consultant for Abbott. Alain Fraisse is a consultant for Abbott. Osman Baspinar is a consultant for Abbott. Evan Zahn is a consultant/proctor for Abbott, Edwards, Medtronic, National PI ADO II AS IDE Trial and Alterra/S3.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

