Salt-Inducible Kinase 3 Promotes Vascular Smooth Muscle Cell Proliferation and Arterial Restenosis by Regulating AKT and PKA-CREB Signaling
- PMID: 34196217
- PMCID: PMC8411910
- DOI: 10.1161/ATVBAHA.121.316219
Salt-Inducible Kinase 3 Promotes Vascular Smooth Muscle Cell Proliferation and Arterial Restenosis by Regulating AKT and PKA-CREB Signaling
Abstract
Objective: Arterial restenosis is the pathological narrowing of arteries after endovascular procedures, and it is an adverse event that causes patients to experience recurrent occlusive symptoms. Following angioplasty, vascular smooth muscle cells (SMCs) change their phenotype, migrate, and proliferate, resulting in neointima formation, a hallmark of arterial restenosis. SIKs (salt-inducible kinases) are a subfamily of the AMP-activated protein kinase family that play a critical role in metabolic diseases including hepatic lipogenesis and glucose metabolism. Their role in vascular pathological remodeling, however, has not been explored. In this study, we aimed to understand the role and regulation of SIK3 in vascular SMC migration, proliferation, and neointima formation.
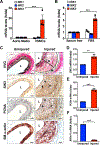
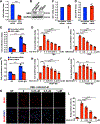
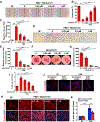
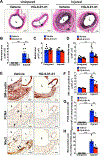
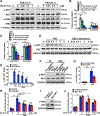
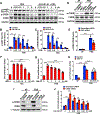
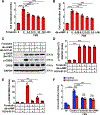
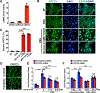
Approach and results: We observed that SIK3 expression was low in contractile aortic SMCs but high in proliferating SMCs. It was also highly induced by growth medium in vitro and in neointimal lesions in vivo. Inactivation of SIKs significantly attenuated vascular SMC proliferation and up-regulated p21CIP1 and p27KIP1. SIK inhibition also suppressed SMC migration and modulated actin polymerization. Importantly, we found that inhibition of SIKs reduced neointima formation and vascular inflammation in a femoral artery wire injury model. In mechanistic studies, we demonstrated that inactivation of SIKs mainly suppressed SMC proliferation by down-regulating AKT (protein kinase B) and PKA (protein kinase A)-CREB (cAMP response element-binding protein) signaling. CRTC3 (CREB-regulated transcriptional coactivator 3) signaling likely contributed to SIK inactivation-mediated antiproliferative effects.
Conclusions: These findings suggest that SIK3 may play a critical role in regulating SMC proliferation, migration, and arterial restenosis. This study provides insights into SIK inhibition as a potential therapeutic strategy for treating restenosis in patients with peripheral arterial disease.
Keywords: cell proliferation; inflammation; neointima; phenotype; vascular remodeling.
Figures


Comment in
-
SIKs (Salt-Inducible Kinases) in Arterial Restenosis.Arterioscler Thromb Vasc Biol. 2021 Sep;41(9):2452-2453. doi: 10.1161/ATVBAHA.121.316691. Epub 2021 Jul 29. Arterioscler Thromb Vasc Biol. 2021. PMID: 34320836 No abstract available.
References
-
- Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, Schlager O, Cejna M, Lammer J and Minar E. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354:1879–88. - PubMed
-
- Owens GK, Kumar MS and Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. - PubMed
-
- Jukema JW, Verschuren JJ, Ahmed TA and Quax PH. Restenosis after PCI. Part 1: pathophysiology and risk factors. Nat Rev Cardiol. 2012;9:53–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

