Influenza vaccination in the elderly boosts antibodies against conserved viral proteins and egg-produced glycans
- PMID: 34196304
- PMCID: PMC8245176
- DOI: 10.1172/JCI148763
Influenza vaccination in the elderly boosts antibodies against conserved viral proteins and egg-produced glycans
Abstract
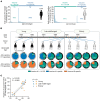
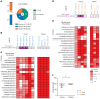
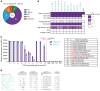
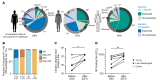
Seasonal influenza vaccination elicits a diminished adaptive immune response in the elderly, and the mechanisms of immunosenescence are not fully understood. Using Ig-Seq, we found a marked increase with age in the prevalence of cross-reactive (CR) serum antibodies that recognize both the H1N1 (vaccine-H1) and H3N2 (vaccine-H3) components of an egg-produced split influenza vaccine. CR antibodies accounted for 73% ± 18% of the serum vaccine responses in a cohort of elderly donors, 65% ± 15% in late middle-aged donors, and only 13% ± 5% in persons under 35 years of age. The antibody response to non-HA antigens was boosted by vaccination. Recombinant expression of 19 vaccine-H1+H3 CR serum monoclonal antibodies (s-mAbs) revealed that they predominantly bound to non-HA influenza proteins. A sizable fraction of vaccine-H1+H3 CR s-mAbs recognized with high affinity the sulfated glycans, in particular sulfated type 2 N-acetyllactosamine (Galβ1-4GalNAcβ), which is found on egg-produced proteins and thus unlikely to contribute to protection against influenza infection in humans. Antibodies against sulfated glycans in egg-produced vaccine had been identified in animals but were not previously characterized in humans. Collectively, our results provide a quantitative basis for how repeated exposure to split influenza vaccine correlates with unintended focusing of serum antibody responses to non-HA antigens that may result in suboptimal immunity against influenza.
Keywords: Adaptive immunity; Infectious disease; Influenza; Vaccines.
Conflict of interest statement
Figures

Comment in
-
Decreased vaccine protection of egg-based influenza vaccine in the elderly and nonhemagglutinin-focused immunity.J Clin Invest. 2021 Aug 2;131(15):e151732. doi: 10.1172/JCI151732. J Clin Invest. 2021. PMID: 34338229 Free PMC article.

