Safety and Persistence of Nalmefene Treatment for Alcohol Dependence. Results from Two Post-authorisation Safety Studies
- PMID: 34196359
- PMCID: PMC8406067
- DOI: 10.1093/alcalc/agab045
Safety and Persistence of Nalmefene Treatment for Alcohol Dependence. Results from Two Post-authorisation Safety Studies
Abstract
Aims: Two post-authorisation studies assessed the safety and persistence of patients' use of nalmefene.
Methods: The START study (EUPAS5678) was a non-interventional, multi-country, prospective, 18-month (8 follow-up visits) cohort study including outpatients initiating nalmefene for the first time. The multi-database retrospective cohort study (MDRC, EUPAS14083) included baseline and follow-up data from German, Swedish and UK healthcare databases. Both studies permitted 'all comers' without explicit exclusion criteria; predefined subgroups of interest included the elderly (≥65 years) as well as patients with significant psychiatric and/or somatic comorbidities.
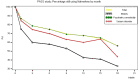
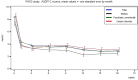
Results: START study: Overall, the mean duration of nalmefene treatment was 10.3 ± 7.3 months (N = 1348), with 49.0% of patients treated for ≥1 year; frequent reasons for treatment discontinuation were 'goal reached' and 'drug cost'. The most frequently reported adverse drug reactions (ADRs) were nausea (4.7%), dizziness (3.2%) and insomnia (2.0%). ADR rates appeared higher in the elderly subpopulation (18.6% reported ≥1 ADR vs. 12.0% in the total population) but were not higher in the other predefined subgroups.MDRC study: The database follow-up analysis followed 2892 patients over 18 months for whom the duration of nalmefene treatment was between 2 and 3 months and <5% of patients used nalmefene for ≥1 year.
Conclusions: Despite the inclusion of a wider patient population (e.g. elderly patients and those with relevant co-morbidities), the safety and tolerability profile of nalmefene given in routine practice was consistent with previous clinical studies. The differing rates of persistence beyond 1 year likely reflect the different methodologies and highlight the relevance of psychosocial support at follow-up visits.
© The Author(s) 2021. Medical Council on Alcohol and Oxford University Press.
Figures
References
-
- Selincro . (2018) Summary of product characteristics. Valby, Denmark: H. Lundbeck A/S.
-
- Allan CA, Smith I, Mellin M. (2002) Changes in psychological symptoms during ambulant detoxification. Alcohol Alcohol 37:241–4. - PubMed
-
- Ambrogne JA. (2002) Reduced-risk drinking as a treatment goal: What clinicians need to know. J Subst Abuse Treat 22:45–53. - PubMed
-
- Atkinson RM. (1990) Aging and alcohol use disorders: diagnostic issues in the elderly. Int Psychogeriatr 2:55–72. - PubMed
-
- Bradley KA, DeBenedetti AF, Volk RJ, et al. (2007) AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res 31:1208–17. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

