Polatuzumab vedotin as a salvage and bridging treatment in relapsed or refractory large B-cell lymphomas
- PMID: 34196677
- PMCID: PMC8288676
- DOI: 10.1182/bloodadvances.2020004155
Polatuzumab vedotin as a salvage and bridging treatment in relapsed or refractory large B-cell lymphomas
Abstract
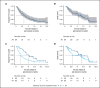
The antibody-drug conjugate polatuzumab vedotin (pola) has recently been approved in combination with bendamustine and rituximab (pola-BR) for patients with refractory or relapsed (r/r) large B-cell lymphoma (LBCL). To investigate the efficacy of pola-BR in a real-world setting, we retrospectively analyzed 105 patients with LBCL who were treated in 26 German centers under the national compassionate use program. Fifty-four patients received pola as a salvage treatment and 51 patients were treated with pola with the intention to bridge to chimeric antigen receptor (CAR) T-cell therapy (n = 41) or allogeneic hematopoietic cell transplantation (n = 10). Notably, patients in the salvage and bridging cohort had received a median of 3 prior treatment lines. In the salvage cohort, the best overall response rate was 48.1%. The 6-month progression-free survival and overall survival (OS) was 27.7% and 49.6%, respectively. In the bridging cohort, 51.2% of patients could be successfully bridged with pola to the intended CAR T-cell therapy. The combination of pola bridging and successful CAR T-cell therapy resulted in a 6-month OS of 77.9% calculated from pola initiation. Pola vedotin-rituximab without a chemotherapy backbone demonstrated encouraging overall response rates up to 40%, highlighting both an appropriate alternative for patients unsuitable for chemotherapy and a new treatment option for bridging before leukapheresis in patients intended for CAR T-cell therapy. Furthermore, 7 of 12 patients with previous failure of CAR T-cell therapy responded to a pola-containing regimen. These findings suggest that pola may serve as effective salvage and bridging treatment of r/r LBCL patients.
© 2021 by The American Society of Hematology.
Figures



References
-
- Van Den Neste E, Schmitz N, Mounier N, et al. . Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant. 2016;51(1):51-57. - PubMed
-
- González-Barca E, Boumendil A, Blaise D, et al. . Outcome in patients with diffuse large B-cell lymphoma who relapse after autologous stem cell transplantation and receive active therapy. A retrospective analysis of the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2020;55(2):393-399. - PubMed

