Monoallelic IRF5 deficiency in B cells prevents murine lupus
- PMID: 34197340
- PMCID: PMC8410043
- DOI: 10.1172/jci.insight.141395
Monoallelic IRF5 deficiency in B cells prevents murine lupus
Abstract
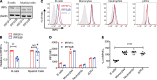
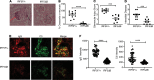
Gain-of-function polymorphisms in the transcription factor IFN regulatory factor 5 (IRF5) are associated with an increased risk of developing systemic lupus erythematosus. However, the IRF5-expressing cell type(s) responsible for lupus pathogenesis in vivo is not known. We now show that monoallelic IRF5 deficiency in B cells markedly reduced disease in a murine lupus model. In contrast, similar reduction of IRF5 expression in macrophages, monocytes, and neutrophils did not reduce disease severity. B cell receptor and TLR7 signaling synergized to promote IRF5 phosphorylation and increase IRF5 protein expression, with these processes being independently regulated. This synergy increased B cell-intrinsic IL-6 and TNF-α production, both key requirements for germinal center (GC) responses, with IL-6 and TNF-α production in vitro and in vivo being substantially lower with loss of 1 allele of IRF5. Mechanistically, TLR7-dependent IRF5 nuclear translocation was reduced in B cells from IRF5-heterozygous mice. In addition, we show in multiple lupus models that IRF5 expression was dynamically regulated in vivo with increased expression in GC B cells compared with non-GC B cells and with further sequential increases during progression to plasmablasts and long-lived plasma cells. Overall, a critical threshold level of IRF5 in B cells was required to promote disease in murine lupus.
Keywords: Autoimmune diseases; Autoimmunity.
Conflict of interest statement
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

