Sales of antibiotics and hydroxychloroquine in India during the COVID-19 epidemic: An interrupted time series analysis
- PMID: 34197449
- PMCID: PMC8248656
- DOI: 10.1371/journal.pmed.1003682
Sales of antibiotics and hydroxychloroquine in India during the COVID-19 epidemic: An interrupted time series analysis
Abstract
Background: We assessed the impact of the coronavirus disease 2019 (COVID-19) epidemic in India on the consumption of antibiotics and hydroxychloroquine (HCQ) in the private sector in 2020 compared to the expected level of use had the epidemic not occurred.
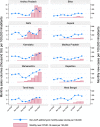
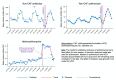
Methods and findings: We performed interrupted time series (ITS) analyses of sales volumes reported in standard units (i.e., doses), collected at regular monthly intervals from January 2018 to December 2020 and obtained from IQVIA, India. As children are less prone to develop symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, we hypothesized a predominant increase in non-child-appropriate formulation (non-CAF) sales. COVID-19-attributable changes in the level and trend of monthly sales of total antibiotics, azithromycin, and HCQ were estimated, accounting for seasonality and lockdown period where appropriate. A total of 16,290 million doses of antibiotics were sold in India in 2020, which is slightly less than the amount in 2018 and 2019. However, the proportion of non-CAF antibiotics increased from 72.5% (95% CI: 71.8% to 73.1%) in 2019 to 76.8% (95% CI: 76.2% to 77.5%) in 2020. Our ITS analyses estimated that COVID-19 likely contributed to 216.4 million (95% CI: 68.0 to 364.8 million; P = 0.008) excess doses of non-CAF antibiotics and 38.0 million (95% CI: 26.4 to 49.2 million; P < 0.001) excess doses of non-CAF azithromycin (equivalent to a minimum of 6.2 million azithromycin treatment courses) between June and September 2020, i.e., until the peak of the first epidemic wave, after which a negative change in trend was identified. In March 2020, we estimated a COVID-19-attributable change in level of +11.1 million doses (95% CI: 9.2 to 13.0 million; P < 0.001) for HCQ sales, whereas a weak negative change in monthly trend was found for this drug. Study limitations include the lack of coverage of the public healthcare sector, the inability to distinguish antibiotic and HCQ sales in inpatient versus outpatient care, and the suboptimal number of pre- and post-epidemic data points, which could have prevented an accurate adjustment for seasonal trends despite the robustness of our statistical approaches.
Conclusions: A significant increase in non-CAF antibiotic sales, and particularly azithromycin, occurred during the peak phase of the first COVID-19 epidemic wave in India, indicating the need for urgent antibiotic stewardship measures.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: MP is a member of the Editorial Board of PLOS Medicine, he coedits the PLOS Tuberculosis Channel and is the Editor-in-Chief of PLoS Global Public Health. All the other authors have no conflicts of interest to declare.
Figures


References
-
- Indian Council of Medical Research. Treatment guidelines for antimicrobial use in common syndromes. New Delhi: Indian Council of Medical Research; 2019.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

