Anti-inflammatory, antibacterial and immunomodulatory treatment in children with symptoms corresponding to the research condition PANS (Pediatric Acute-onset Neuropsychiatric Syndrome): A systematic review
- PMID: 34197525
- PMCID: PMC8248649
- DOI: 10.1371/journal.pone.0253844
Anti-inflammatory, antibacterial and immunomodulatory treatment in children with symptoms corresponding to the research condition PANS (Pediatric Acute-onset Neuropsychiatric Syndrome): A systematic review
Abstract
Objective: To assess effects of treatment against a hypothesized neuroinflammation in children with symptoms corresponding to the research condition Pediatric Acute-onset Neuropsychiatric Syndrome (PANS) which is not included in current diagnostic systems.
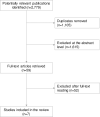
Methods: Systematic literature searches were performed (1998 to June 2020) in PubMed, Embase, the Cochrane Library, CINAHL, PsycInfo, and HTA databases. Inclusion criteria: patients (P) were children (<18 years) with PANS; intervention (I)/comparison (C) was use of, versus no use of, anti-inflammatory, antibacterial or immunomodulating treatments; outcomes (O) were health-related quality of life (HRQL), level of functioning, symptom change, and complications.
Results: Four randomised controlled trials (RCTs) and three non-RCTs, including 23 to 98 patients, fulfilled the PICO. HRQL was not investigated in any study. Regarding level of functioning, two RCTs investigated antibiotics (penicillin V, azithromycin) and one RCT investigated immunomodulating treatments (intravenous immunoglobulins (IVIG), plasma exchange). Regarding symptoms, two non-RCTs investigated anti-inflammatory treatment (cyclooxygenase (COX) inhibitors, corticosteroids), two RCTs and one non-RCT investigated antibiotics (penicillin V, azithromycin), and two RCTs investigated immunomodulating treatments (IVIG, plasma exchange). Complications, reported in five studies, were consistent with those listed in the summary of products characteristics (SPC). All studies were assessed to have some or major problems regarding directness, the absence of an established diagnosis contributing to clinical diversity in the studied populations. All studies were assessed to have major risk of bias, including selection and detection biases. Due to clinical and methodological diversity, meta-analyses were not performed.
Conclusion: This systematic review reveals very low certainty of evidence of beneficial effects, and moderate certainty of evidence of adverse effects, of anti-inflammatory, antibacterial or immunomodulating treatments in patients with symptoms corresponding to the research condition PANS. Available evidence neither supports nor excludes potential beneficial effects, but supports that such treatment can result in adverse effects.
Registration: PROSPERO (CRD42020155714).
Conflict of interest statement
M.J. and E.F. work at the Child Neuropsychiatry Centre and the Gillberg Neuropsychiatry Centre, to which referrals of patients occur, for assessments and treatment, when the research condition PANS/PANDAS is suspected. M. J. has received research grants from Shire and has been engaged as a speaker or consultant by Eli Lilly, Shire, Ginsana, PCM Scientific, Evolan, and New Nordic, all unrelated to the present work. S.E., P.H., C.W., and S.M.W. declare no competing interests. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- National Board of Health and Welfare National guidelines for care of depression and anxiety [Nationella riktlinjer för vård vid depression och ångestsyndrom: stöd för styrning och ledning]. Available from: https://wwwsocialstyrelsense/globalassets/sharepoint-dokument/artikelkat....
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

