Identification of a critical lipid ratio in raft-like phases exposed to nitric oxide: An AFM study
- PMID: 34197799
- PMCID: PMC8390956
- DOI: 10.1016/j.bpj.2021.06.009
Identification of a critical lipid ratio in raft-like phases exposed to nitric oxide: An AFM study
Abstract
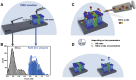
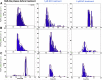
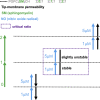
Lipid rafts are discrete, heterogeneous domains of phospholipids, sphingolipids, and sterols that are present in the cell membrane. They are responsible for conducting cell signaling and maintaining lipid-protein functionality. Redox-stress-induced modifications to any of their components can severely alter the mechanics and dynamics of the membrane causing impairment to the lipid-protein functionality. Here, we report on the effect of sphingomyelin (SM) in controlling membrane permeability and its role as a regulatory lipid in the presence of nitric oxide (NO). Force spectroscopy and atomic force microscopy imaging of raft-like phases (referring here to the coexistence of "liquid-ordered" and "liquid-disordered" phases in model bilayer membranes) prepared from lipids: 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC):SM:cholesterol (CH) (at three ratios) showed that the adhesion forces to pull the tip out of the membrane increased with increasing SM concentration, indicating decreased membrane permeability. However, in the presence of NO radical (1 and 5 μM), the adhesion forces decreased depending on SM concentration. The membrane was found to be stable at the ratio POPC:SM:CH (2:1:1) even when exposed to 1 μM NO. We believe that this is a critical ratio needed by the raft-like phases to maintain homeostasis under stress conditions. The stability could be due to an interplay existing between SM and CH. However, at 5 μM NO, membrane deteriorations were detected. For POPC:SM:CH (2:2:1) ratio, NO displayed a pro-oxidant behavior and damaged the membrane at both radical concentrations. These changes were reflected by the differences in the height profiles of the raft-like phases observed by atomic force microscopy imaging. Malondialdehyde (a peroxidation product) detection suggests that lipids may have undergone lipid nitroxidation. The changes were instantaneous and independent of radical concentration and incubation time. Our study underlines the need for identifying appropriate ratios in the lipid rafts of the cell membranes to withstand redox imbalances caused by radicals such as NO.
Copyright © 2021 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- van Meer G., de Kroon A.I. Lipid map of the mammalian cell. J. Cell Sci. 2011;124:5–8. - PubMed
-
- Karanth S., Meesaragandla B., Delcea M. Changing surface properties of artificial lipid membranes at the interface with biopolymer coated gold nanoparticles under normal and redox conditions. Biophys. Chem. 2020;267:106465. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

