Evaluation of spike protein antigens for SARS-CoV-2 serology
- PMID: 34197839
- PMCID: PMC8239204
- DOI: 10.1016/j.jviromet.2021.114222
Evaluation of spike protein antigens for SARS-CoV-2 serology
Abstract
Background: Spike protein domains are being used in various serology-based assays to detect prior exposure to SARS-CoV-2 virus. However, there has been limited comparison of antibody titers against various spike protein antigens among COVID-19 infected patients.
Methods: We compared four spike proteins (RBD, S1, S2 and a stabilized spike trimer (ST)) representing commonly used antigens for their reactivity to human IgG antibodies using indirect ELISA in serum from COVID-19 patients and pre-2020 samples. ST ELISA was also compared against the EUROIMMUN IgG ELISA test. Further, we estimated time appropriate IgG and IgA seropositivity rates in COVID-19 patients using a panel of sera samples collected longitudinally from the day of onset of symptoms (DOS).
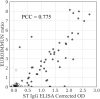
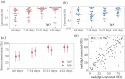
Results: Among the four spike antigens tested, the ST demonstrated the highest sensitivity (86.2 %; 95 % CI: 77.8-91.7 %), while all four antigens showed high specificity to COVID-19 sera (94.7-96.8 %). 13.8 % (13/94) of the samples did not show seroconversion in any of the four antigen-based assays. In a double-blinded head-to-head comparison, ST based IgG ELISA displayed a better sensitivity (87.5 %, 95 % CI: 76.4-93.8 %) than the EUROIMMUN IgG ELISA (67.9 %, 95 % CI: 54.8-78.6 %). Further, in ST-based assays, we found 48 % and 50 % seroconversion in the first six days (from DOS) for IgG and IgA antibodies, respectively, which increased to 84 % (IgG) and 85 % (IgA) for samples collected ≥22 days from DOS.
Conclusions: Comparison of spike antigens demonstrates that spike trimer protein is a superior option as an ELISA antigen for COVID-19 serology.
Keywords: SARS-CoV-2; Serology; Spike trimer ELISA.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

References
-
- Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M., Jiang K., Arunkumar G.A., Jurczyszak D., Polanco J., Bermudez-Gonzalez M., Kleiner G., Aydillo T., Miorin L., Fierer D.S., Lugo L.A., Kojic E.M., Stoever J., Liu S.T.H., Cunningham-Rundles C., Felgner P.L., Moran T., García-Sastre A., Caplivski D., Cheng A.C., Kedzierska K., Vapalahti O., Hepojoki J.M., Simon V., Krammer F. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020;26:1033–1036. doi: 10.1038/s41591-020-0913-5. - DOI - PMC - PubMed
-
- Brochot E., Demey B., Touzé A., Belouzard S., Dubuisson J., Schmit J.L., Duverlie G., Francois C., Castelain S., Helle F. Anti-spike, anti-nucleocapsid and neutralizing antibodies in SARS-CoV-2 inpatients and asymptomatic individuals. Front. Microbiol. 2020;11:2468. doi: 10.3389/fmicb.2020.584251. - DOI - PMC - PubMed
-
- Brown L.D., Cai T.T., das Gupta A. Interval estimation for a binomial proportion. Stat. Sci. 2001;16:101–117. doi: 10.1214/ss/1009213286. - DOI
-
- Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., Grifoni A., Ramirez S.I., Haupt S., Frazier A., Nakao C., Rayaprolu V., Rawlings S.A., Peters B., Krammer F., Simon V., Saphire E.O., Smith D.M., Weiskopf D., Sette A., Crotty S. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021 doi: 10.1126/science.abf4063. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

