Dual-targeted anti-CMV/anti-HIV-1 heterodimers
- PMID: 34197866
- PMCID: PMC8458242
- DOI: 10.1016/j.biochi.2021.06.011
Dual-targeted anti-CMV/anti-HIV-1 heterodimers
Abstract
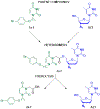
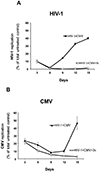
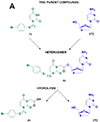
Despite the development of efficient anti-human immunodeficiency virus-1 (HIV-1) therapy, HIV-1 associated pathogens remain a major clinical problem. Human cytomegalovirus (CMV) is among the most common HIV-1 copathogens and one of the main causes of persistent immune activation associated with dysregulation of the immune system, cerebrovascular and cardiovascular pathologies, and premature aging. Here, we report on the development of dual-targeted drugs with activity against both HIV-1 and CMV. We synthesized seven compounds that constitute conjugates of molecules that suppress both pathogens. We showed that all seven compounds exhibit low cytotoxicity and efficiently inhibited both viruses in cell lines. Furthermore, we chose a representative compound and demonstrated that it efficiently suppressed replication of HIV-1 and CMV in human lymphoid tissue ex vivo coinfected with both viruses. Further development of such compounds may lead to the development of dual-targeted anti-CMV/HIV-1 drugs.
Keywords: CMV; HIV-1; Heterodimers; Human tissues; Viral diseases.
Copyright © 2021 Elsevier B.V. and Société Française de Biochimie et Biologie Moléculaire (SFBBM). All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


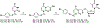
References
-
- Lichtner M, Cicconi P, Vita S, Cozzi-Lepri A, Galli M, Lo Caputo S, Saracino A, De Luca A, Moioli M, Maggiolo F, Marchetti G, Vullo V, d'Arminio Monforte A, Study IF, Cytomegalovirus coinfection is associated with an increased risk of severe non-AIDS-defining events in a large cohort of HIV-infected patients, J Infect Dis, 211 (2015) 178–186. 10.1093/infdis/jiu417. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

