Isocratic reporter-exclusion immunoassay using restricted-access adsorbents
- PMID: 34198311
- PMCID: PMC9798887
- DOI: 10.1039/d1an00396h
Isocratic reporter-exclusion immunoassay using restricted-access adsorbents
Abstract
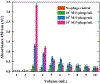
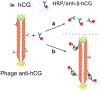
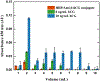
We introduce analyte-dependent exclusion of reporter reagents from restricted-access adsorbents as the basis of an isocratic reporter-exclusion immunoassay for viruses, proteins, and other analytes. Capto™ Core 700 and related resins possess a noninteracting size-selective outer layer surrounding a high-capacity nonspecific mixed-mode capture adsorbent core. In the absence of analyte, antibody-enzyme reporter conjugates can enter the adsorbent and be captured, and their signal is lost. In the presence of large or artificially-expanded analytes, reporter reagents bind to analyte species to form complexes large enough to be excluded from the adsorbent core, allowing their signal to be observed. This assay principle is demonstrated using M13 bacteriophage virus and human chorionic gonadotropin as model analytes. The simple isocratic detection approach described here allows a rapid implementation of immunoassay for detection of a wide range of analytes and uses inexpensive, generally-applicable, and stable column materials instead of costly analyte-specific immunoaffinity adsorbents.
Conflict of interest statement
Conflicts of Interest
There are no conflicts to declare.
Figures
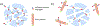

References
-
- Maragos CM, Recent Advances in the Development of Novel Materials for Mycotoxin Analysis, Anal. Bioanal. Chem, 2009, 395, 1205–1213. - PubMed
-
- Pastor-Navarro N, Maquieira A, and Puchades R, Review on Immunoanalytical Determination of Tetracycline and Sulfonamide Residues in Edible Products, Anal. Bioanal. Chem, 2009, 395, 907–920. - PubMed
-
- Prieto-Simon B, Noguer T, and Campàs M, Emerging Biotools for Assessment of Mycotoxins in the Past Decade, TrAC, Trends Anal. Chem, 2007, 26, 689–702.
-
- Herman J and Shushan B, Tandem Mass Spectrometry – Applications and Principles, ed. Prasain J, InTech, Rijeka, 2012, Clinical applications, 673–720.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

