Beneficial Insects Deliver Plant Growth-Promoting Bacterial Endophytes between Tomato Plants
- PMID: 34198479
- PMCID: PMC8231829
- DOI: 10.3390/microorganisms9061294
Beneficial Insects Deliver Plant Growth-Promoting Bacterial Endophytes between Tomato Plants
Abstract
Beneficial insects and mites, including generalist predators of the family Miridae, are widely used in biocontrol programs against many crop pests, such as whiteflies, aphids, lepidopterans and mites. Mirid predators frequently complement their carnivore diet by feeding plant sap with their piercing-sucking mouthparts. This implies that mirids may act as vectors of phytopathogenic and beneficial microorganisms, such as plant growth-promoting bacterial endophytes. This work aimed at understanding the role of two beneficial mirids (Macrolophus pygmaeus and Nesidiocoris tenuis) in the acquisition and transmission of two plant growth-promoting bacteria, Paraburkholderia phytofirmans strain PsJN (PsJN) and Enterobacter sp. strain 32A (32A). Both bacterial strains were detected on the epicuticle and internal body of both mirids at the end of the mirid-mediated transmission. Moreover, both mirids were able to transmit PsJN and 32A between tomato plants and these bacterial strains could be re-isolated from tomato shoots after mirid-mediated transmission. In particular, PsJN and 32A endophytically colonised tomato plants and moved from the shoots to roots after mirid-mediated transmission. In conclusion, this study provided novel evidence for the acquisition and transmission of plant growth-promoting bacterial endophytes by beneficial mirids.
Keywords: Macrolophus pygmaeus; Nesidiocoris tenuis; beneficial mirids; plant growth-promoting bacterial endophytes.
Conflict of interest statement
N.G. and F.W. are employed by Biobest NV. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
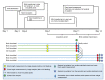


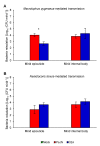
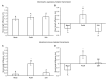
References
-
- Pertot I., Caffi T., Rossi V., Mugnai L., Hoffmann C., Grando M., Gary C., Lafond D., Duso C., Thiery D., et al. A critical review of plant protection tools for reducing pesticide use on grapevine and new perspectives for the implementation of IPM in viticulture. Crop Prot. 2017;97:70–84. doi: 10.1016/j.cropro.2016.11.025. - DOI
-
- Calvo J., Bolckmans K., Stansly P.A., Urbaneja A. Predation by Nesidiocoris tenuis on Bemisia tabaci and injury to tomato. BioControl. 2009;54:237–246. doi: 10.1007/s10526-008-9164-y. - DOI
-
- Urbaneja A., Montón H., Mollá O. Suitability of the tomato borer Tuta absoluta as prey for Macrolophus pygmaeus and Nesidiocoris tenuis. J. Appl. Èntomol. 2009;133:292–296. doi: 10.1111/j.1439-0418.2008.01319.x. - DOI
-
- Castañé C., Arnó J., Gabarra R., Alomar O. Plant damage to vegetable crops by zoophytophagous mirid predators. Biol. Control. 2011;59:22–29. doi: 10.1016/j.biocontrol.2011.03.007. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

