Therapy Development by Genome Editing of Hematopoietic Stem Cells
- PMID: 34198536
- PMCID: PMC8231983
- DOI: 10.3390/cells10061492
Therapy Development by Genome Editing of Hematopoietic Stem Cells
Abstract
Accessibility of hematopoietic stem cells (HSCs) for the manipulation and repopulation of the blood and immune systems has placed them at the forefront of cell and gene therapy development. Recent advances in genome-editing tools, in particular for clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein (Cas) and CRISPR/Cas-derived editing systems, have transformed the gene therapy landscape. Their versatility and the ability to edit genomic sequences and facilitate gene disruption, correction or insertion, have broadened the spectrum of potential gene therapy targets and accelerated the development of potential curative therapies for many rare diseases treatable by transplantation or modification of HSCs. Ongoing developments seek to address efficiency and precision of HSC modification, tolerability of treatment and the distribution and affordability of corresponding therapies. Here, we give an overview of recent progress in the field of HSC genome editing as treatment for inherited disorders and summarize the most significant findings from corresponding preclinical and clinical studies. With emphasis on HSC-based therapies, we also discuss technical hurdles that need to be overcome en route to clinical translation of genome editing and indicate advances that may facilitate routine application beyond the most common disorders.
Keywords: CRISPR/Cas; TALEN; ZFN; base editor; blood disorders; gene therapy (GT); genome editing; hematopoietic stem cell; monogenic disorder; prime editor.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
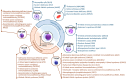
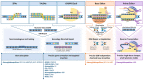
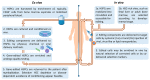
References
-
- Duarte R.F., Labopin M., Bader P., Basak G.W., Bonini C., Chabannon C., Corbacioglu S., Dreger P., Dufour C. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: Current practice in Europe, 2019. Bone Marrow Transplant. 2019;54:1525–1552. doi: 10.1038/s41409-019-0516-2. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

