The BMP Pathway in Blood Vessel and Lymphatic Vessel Biology
- PMID: 34198654
- PMCID: PMC8232321
- DOI: 10.3390/ijms22126364
The BMP Pathway in Blood Vessel and Lymphatic Vessel Biology
Abstract
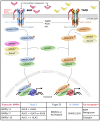
Bone morphogenetic proteins (BMPs) were originally identified as the active components in bone extracts that can induce ectopic bone formation. In recent decades, their key role has broadly expanded beyond bone physiology and pathology. Nowadays, the BMP pathway is considered an important player in vascular signaling. Indeed, mutations in genes encoding different components of the BMP pathway cause various severe vascular diseases. Their signaling contributes to the morphological, functional and molecular heterogeneity among endothelial cells in different vessel types such as arteries, veins, lymphatic vessels and capillaries within different organs. The BMP pathway is a remarkably fine-tuned pathway. As a result, its signaling output in the vessel wall critically depends on the cellular context, which includes flow hemodynamics, interplay with other vascular signaling cascades and the interaction of endothelial cells with peri-endothelial cells and the surrounding matrix. In this review, the emerging role of BMP signaling in lymphatic vessel biology will be highlighted within the framework of BMP signaling in the circulatory vasculature.
Keywords: BMP; BMP pathway fine-tuning; lymphatic vessel biology; mechano-transduction; signaling cross-talk; vascular malformations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

