HER2-Low Breast Cancer: Molecular Characteristics and Prognosis
- PMID: 34198891
- PMCID: PMC8201345
- DOI: 10.3390/cancers13112824
HER2-Low Breast Cancer: Molecular Characteristics and Prognosis
Abstract
Background: We aimed to determine the distribution of intrinsic subtypes within HER2-low breast cancer (BC), and to describe the prognostic impact of HER2-low status on survival outcomes.
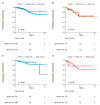
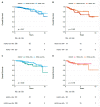
Methods: This is a retrospective, observational study of primary BC extracted from The Cancer Genome Atlas dataset. We described the distribution of PAM50 intrinsic subtypes within HER2-low BC subtype according to hormonal receptor status (positive (HR+) and negative (HR-)). Secondly, we assessed the impact of HER2-low on survival outcomes (progression-free interval (PFI), disease-free interval (DFI), and overall survival (OS)).
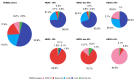
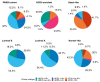
Results: We analyzed 804 primary BCs, including 410 (51%) HER2-low BCs (336 HR+ and 74 HR-). The proportion of HER2-enriched tumors was higher in the HER2-low/HR- group compared to HER2-low/HR+ (13.7% versus 1.2%, respectively). HER2-enriched tumors were more frequent in HER2-low/HR- and HER2-low/HR+ subtypes, compared to HER2-negative/HR- and HER2-negative/HR+ subtypes, respectively (13.7% versus 1.6% and 1.2% versus 0.5%, respectively). We observed no significant differences in PFI, DFI, and OS between HER2-low subtypes and each non-HER2-low subtype paired by HR status.
Conclusions: Our characterization of PAM50 intrinsic subtypes within HER2-low breast cancer may explain the different clinical behaviors and responses to treatment, and ultimately support further investigation of new treatment strategies in the HER2-low category. Moreover, it highlights the importance of considering HR status in the HER2-low category.
Keywords: HER2-low; PAM50; TCGA; breast cancer; prognosis.
Conflict of interest statement
Elisa Agostinetto, Mattia Rediti, Danai Fimereli and Véronique Debien have no conflicts of interests. Martine Piccart: Board Member (Scientific Board): Oncolytics; Consultant (honoraria): AstraZeneca, Camel-IDS, Crescendo Biologics, G1 Therapeutics, Genentech, Huya, Immunomedics, Lilly, Menarini, MSD, Novartis, Odonate, Oncolytics, Periphagen, Pfizer, Roche, Seattle Genetics, Immutep, NBE Therapeutics, SeaGen; Research grants to her Institute: AstraZeneca, Lilly, MSD, Novartis, Pfizer, Radius, Roche-Genentech, Servier, Synthon (outside the submitted work). Philippe Aftimos: Consulting: Boehringer Ingelheim, Macrogenics, Roche, Novartis, Amcure, Servier, G1 Therapeutics, Radius, Deloitte; Honoraria: Synthon, Amgen, Novartis, Gilead; Travel grants: Amgen, MSD, Pfizer, Roche; Research funding to my institution: Roche (outside the submitted work). Christos Sotiriou: advisory board (receipt of honoraria or consultations fees): Astellas, Cepheid, Vertex, Seattle genetics, Puma, Amgen. Participation in company sponsored speaker’s bureau: Eisai, Prime Oncology, Teva, Foundation Medicine. Other support (travel, accommodation expenses): Roche, Genentech, Pfizer (outside the submitted work) Evandro de Azambuja: honoraria and advisory board: Roche/GNE, Novartis, Seattle Genetics, Zodiacs, Libbs, Pierre Fabre and Lilly; travel grants: Roche/GNE, GSK/Novartis. Research grant for his institute: Roche/GNE, AstraZeneca, Novartis, and Servier (outside the submitted work).
Figures



References
-
- Mendes D., Alves C., Afonso N., Cardoso F., Passos-Coelho J.L., Costa L., Andrade S., Batel-Marques F. The benefit of HER2-targeted therapies on overall survival of patients with metastatic HER2-positive breast cancer-a systematic review. Breast Cancer Res. 2015;17:140. doi: 10.1186/s13058-015-0648-2. - DOI - PMC - PubMed
-
- Swain S.M., Miles D., Kim S.-B., Im Y.-H., Im S.-A., Semiglazov V., Ciruelos E., Schneeweiss A., Loi S., Monturus E., et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519–530. doi: 10.1016/S1470-2045(19)30863-0. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

