Molecular Pathogenesis of Pulmonary Fibrosis, with Focus on Pathways Related to TGF-β and the Ubiquitin-Proteasome Pathway
- PMID: 34198949
- PMCID: PMC8201174
- DOI: 10.3390/ijms22116107
Molecular Pathogenesis of Pulmonary Fibrosis, with Focus on Pathways Related to TGF-β and the Ubiquitin-Proteasome Pathway
Abstract
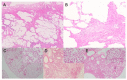
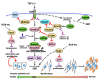
Idiopathic pulmonary fibrosis (IPF) is a progressive and fatal interstitial lung disease. During the past decade, novel pathogenic mechanisms of IPF have been elucidated that have shifted the concept of IPF from an inflammatory-driven to an epithelial-driven disease. Dysregulated repair responses induced by recurrent epithelial cell damage and excessive extracellular matrix accumulation result in pulmonary fibrosis. Although there is currently no curative therapy for IPF, two medications, pirfenidone and nintedanib, have been introduced based on understanding the pathogenesis of the disease. In this review, we discuss advances in understanding IPF pathogenesis, highlighting epithelial-mesenchymal transition (EMT), the ubiquitin-proteasome system, and endothelial cells. TGF-β is a central regulator involved in EMT and pulmonary fibrosis. HECT-, RING finger-, and U-box-type E3 ubiquitin ligases regulate TGF-β-Smad pathway-mediated EMT via the ubiquitin-proteasome pathway. p27 degradation mediated by the SCF-type E3 ligase, Skp2, contributes to the progression of pulmonary fibrosis by promotion of either mesenchymal fibroblast proliferation, EMT, or both. In addition to fibroblasts as key effector cells in myofibroblast differentiation and extracellular matrix deposition, endothelial cells also play a role in the processes of IPF. Endothelial cells can transform into myofibroblasts; therefore, endothelial-mesenchymal transition can be another source of myofibroblasts.
Keywords: E3 ligase; TGF-β; endothelial cell; epithelial mesenchymal transition (EMT); fibroblast; idiopathic pulmonary fibrosis (IPF); ubiquitin proteasome system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- American Thoracic Society. European Respiratory Society American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am. J. Respir. Crit. Care Med. 2002;165:277–304. - PubMed
-
- Raghu G., Collard H.R., Egan J.J., Martinez F.J., Behr J., Brown K.K., Colby T.V., Cordier J.F., Flaherty K.R., Lasky J.A., et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

