Epidemiology of the Rhinovirus (RV) in African and Southeast Asian Children: A Case-Control Pneumonia Etiology Study
- PMID: 34198998
- PMCID: PMC8310211
- DOI: 10.3390/v13071249
Epidemiology of the Rhinovirus (RV) in African and Southeast Asian Children: A Case-Control Pneumonia Etiology Study
Abstract
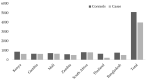
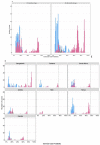
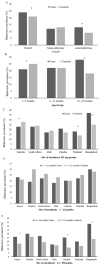
Rhinovirus (RV) is commonly detected in asymptomatic children; hence, its pathogenicity during childhood pneumonia remains controversial. We evaluated RV epidemiology in HIV-uninfected children hospitalized with clinical pneumonia and among community controls. PERCH was a case-control study that enrolled children (1-59 months) hospitalized with severe and very severe pneumonia per World Health Organization clinical criteria and age-frequency-matched community controls in seven countries. Nasopharyngeal/oropharyngeal swabs were collected for all participants, combined, and tested for RV and 18 other respiratory viruses using the Fast Track multiplex real-time PCR assay. RV detection was more common among cases (24%) than controls (21%) (aOR = 1.5, 95%CI:1.3-1.6). This association was driven by the children aged 12-59 months, where 28% of cases vs. 18% of controls were RV-positive (aOR = 2.1, 95%CI:1.8-2.5). Wheezing was 1.8-fold (aOR 95%CI:1.4-2.2) more prevalent among pneumonia cases who were RV-positive vs. RV-negative. Of the RV-positive cases, 13% had a higher probability (>75%) that RV was the cause of their pneumonia based on the PERCH integrated etiology analysis; 99% of these cases occurred in children over 12 months in Bangladesh. RV was commonly identified in both cases and controls and was significantly associated with severe pneumonia status among children over 12 months of age, particularly those in Bangladesh. RV-positive pneumonia was associated with wheezing.
Keywords: PERCH; childhood; epidemiology; pneumonia; rhinovirus.
Conflict of interest statement
S.A.M. has received honoraria for advisory board participation from Bill & Melinda Gates Foundation, Pfizer, Medimmune, and Novartis and institutional grants from GSK, Novartis, Pfizer, Minervax, and Bill & Melinda Gates Foundation and has served on speakers’ bureaus for Sanofi Pasteur and GSK. M.D.K. has received funding for consultation from Merck, Pfizer, and Novartis and grant funding from Merck. C.P. has received grant funding from Merck. K.L.O. has received grant funding from GlaxoSmithKline and Pfizer and participates on technical advisory boards for Merck, Sanofi-Pasteur, PATH, Affinivax, and ClearPath. K.L.K. has received grant funding from Merck Sharp & Dohme. W.A.B. reported funding from Sanofi, PATH, and Bill & Melinda Gates Foundation (BMGF) and contributed to contemporaneous studies from Serum Institute of India, LTD, Roche, and Sanofi. All other authors: No reported conflicts. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Louie J.K., Roy-Burman A., Guardia-LaBar L., Boston E.J., Kiang D., Padilla T., Yagi S., Messenger S., Petru A.M., Glaser C.A., et al. Rhinovirus associated with severe lower respiratory tract infections in children. Pediatr. Infect. Dis. J. 2009;28:337–339. doi: 10.1097/INF.0b013e31818ffc1b. - DOI - PubMed
-
- Esposito S., Daleno C., Prunotto G., Scala A., Tagliabue C., Borzani I., Fossali E., Pelucchi C., Principi N. Impact of viral infections in children with community-acquired pneumonia: Results of a study of 17 respiratory viruses. Influenza Other Respir. Viruses. 2012;7:18–26. doi: 10.1111/j.1750-2659.2012.00340.x. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

