Cholesterol Levels Affect the Performance of AuNPs-Decorated Thermo-Sensitive Liposomes as Nanocarriers for Controlled Doxorubicin Delivery
- PMID: 34199018
- PMCID: PMC8309145
- DOI: 10.3390/pharmaceutics13070973
Cholesterol Levels Affect the Performance of AuNPs-Decorated Thermo-Sensitive Liposomes as Nanocarriers for Controlled Doxorubicin Delivery
Abstract
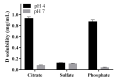
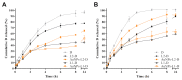
Stimulus-responsive liposomes (L) for triggering drug release to the target site are particularly useful in cancer therapy. This research was focused on the evaluation of the effects of cholesterol levels in the performance of gold nanoparticles (AuNPs)-functionalized L for controlled doxorubicin (D) delivery. Their interfacial and morphological properties, drug release behavior against temperature changes and cytotoxic activity against breast and ovarian cancer cells were studied. Langmuir isotherms were performed to identify the most stable combination of lipid components. Two mole fractions of cholesterol (3.35 mol% and 40 mol%, L1 and L2 series, respectively) were evaluated. Thin-film hydration and transmembrane pH-gradient methods were used for preparing the L and for D loading, respectively. The cationic surface of L allowed the anchoring of negatively charged AuNPs by electrostatic interactions, even inducing a shift in the zeta potential of the L2 series. L exhibited nanometric sizes and spherical shape. The higher the proportion of cholesterol, the higher the drug loading. D was released in a controlled manner by diffusion-controlled mechanisms, and the proportions of cholesterol and temperature of release media influenced its release profiles. D-encapsulated L preserved its antiproliferative activity against cancer cells. The developed liposomal formulations exhibit promising properties for cancer treatment and potential for hyperthermia therapy.
Keywords: Langmuir monolayers; anchoring; anticancer activity; controlled drug release; gold nanoparticles; liposomal formulations; temperature-sensitive nanocarriers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- García M.C., Aloisio C., Onnainty R., Ullio-Gamboa G. In: Self Assembled Nanomaterials in Nanobiomaterials: Nanostructured Materials for Biomedical Applications. 1st ed. Narayan R., editor. Woodhead Publishing; Cambridge, UK: 2017. pp. 41–94.
-
- García M.C. Engineering Drug Delivery Systems. Elsevier; Cambridge, UK: 2019. Nano-And Microparticles as Drug Carriers; pp. 71–110.
Grants and funding
LinkOut - more resources
Full Text Sources

