Preclinical Development of FA5, a Novel AMP-Activated Protein Kinase (AMPK) Activator as an Innovative Drug for the Management of Bowel Inflammation
- PMID: 34199160
- PMCID: PMC8231528
- DOI: 10.3390/ijms22126325
Preclinical Development of FA5, a Novel AMP-Activated Protein Kinase (AMPK) Activator as an Innovative Drug for the Management of Bowel Inflammation
Abstract
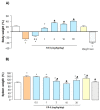
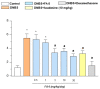
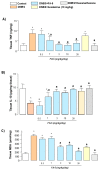
Acadesine (ACA), a pharmacological activator of AMP-activated protein kinase (AMPK), showed a promising beneficial effect in a mouse model of colitis, indicating this drug as an alternative tool to manage IBDs. However, ACA displays some pharmacodynamic limitations precluding its therapeutical applications. Our study was aimed at evaluating the in vitro and in vivo effects of FA-5 (a novel direct AMPK activator synthesized in our laboratories) in an experimental model of colitis in rats. A set of experiments evaluated the ability of FA5 to activate AMPK and to compare the efficacy of FA5 with ACA in an experimental model of colitis. The effects of FA-5, ACA, or dexamethasone were tested in rats with 2,4-dinitrobenzenesulfonic acid (DNBS)-induced colitis to assess systemic and tissue inflammatory parameters. In in vitro experiments, FA5 induced phosphorylation, and thus the activation, of AMPK, contextually to the activation of SIRT-1. In vivo, FA5 counteracted the increase in spleen weight, improved the colon length, ameliorated macroscopic damage score, and reduced TNF and MDA tissue levels in DNBS-treated rats. Of note, FA-5 displayed an increased anti-inflammatory efficacy as compared with ACA. The novel AMPK activator FA-5 displays an improved anti-inflammatory efficacy representing a promising pharmacological tool against bowel inflammation.
Keywords: AMPK; DNBS colitis; immune system; inflammatory bowel diseases; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Vancamelbeke M., Vanuytsel T., Farre R., Verstockt S., Ferrante M., Van Assche G., Rutgeerts P., Schuit F., Vermeire S., Arijs I., et al. Genetic and transcriptomic bases of intestinal epithelial barrier dysfunction in inflammatory bowel disease. Inflamm. Bowel Dis. 2017;23:1718–1729. doi: 10.1097/MIB.0000000000001246. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

