Dried Blood Spot (DBS) Methodology Study for Biomarker Discovery in Lysosomal Storage Disease (LSD)
- PMID: 34199226
- PMCID: PMC8231917
- DOI: 10.3390/metabo11060382
Dried Blood Spot (DBS) Methodology Study for Biomarker Discovery in Lysosomal Storage Disease (LSD)
Abstract
Lysosomal storage diseases (LSDs) are a heterogeneous group of inherited metabolic diseases caused by mutations in genes encoding for proteins involved in the lysosomal degradation of macromolecules. They occur in approximately 1 in 5000 live births and pose a lifelong risk. Therefore, to achieve the maximum benefit from LSDs therapies, a fast and early diagnosis of the disease is required. In this framework, biomarker discovery is a significant factor in disease diagnosis and in predicting its outcomes. On the other hand, the dried blood spot (DBS) based metabolomics platform can open up new pathways for studying non-directional hypothesis approaches to biomarker discovery. This study aims to increase the efficiency of the developed methods for biomarker development in the context of rare diseases, with an improved impact on the reliability of the detected compounds. Thereby, we conducted two independent experiments and integrated them into the screening of the human blood metabolome: (1) comparison of EDTA blood and filter cards in terms of their suitability for metabolomics studies; (2) optimization of the extraction method: a side-by-side comparison of a series of buffers to the best utility to the disease of interest. The findings were compared to previous studies across parameters such as metabolite coverage, sample type suitability, and stability. The results indicate that measurements of metabolites are susceptible to differences in pre-analytical conditions and extraction solvents. This proposed approach can increase the positive rate of the future development of biomarkers. Altogether, the procedure can be easily adapted and applied to other studies, where the limited number of samples is a common barrier.
Keywords: biomarkers; dried blood spot (DBS); lysosomal storage diseases (LSD); mass spectrometry; metabolomics.
Conflict of interest statement
The authors declare no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Figures
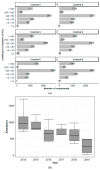

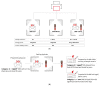
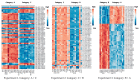


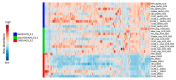
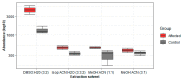
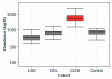
References
-
- Broadhurst D., Goodacre R., Reinke S.N., Kuligowski J., Wilson I.D., Lewis M.R., Dunn W.B. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics. 2018;14:72. doi: 10.1007/s11306-018-1367-3. - DOI - PMC - PubMed
-
- Balashova E., Trifonova O.P., Maslov D.L., Lokhov P.G. Application of dried blood spot for analysis of low molecular weight fraction (metabolome) of blood. Health Prim. Care. 2018;2:1–11. doi: 10.15761/HPC.1000154. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

