Clinical Relevance of Viable Circulating Tumor Cells in Patients with Metastatic Colorectal Cancer: The COLOSPOT Prospective Study
- PMID: 34199250
- PMCID: PMC8231886
- DOI: 10.3390/cancers13122966
Clinical Relevance of Viable Circulating Tumor Cells in Patients with Metastatic Colorectal Cancer: The COLOSPOT Prospective Study
Abstract
Background: Circulating tumor cells (CTCs) allow the real-time monitoring of tumor course and treatment response. This prospective multicenter study evaluates and compares the early predictive value of CTC enumeration with EPISPOT, a functional assay that detects only viable CTCs, and with the CellSearch® system in patients with metastatic colorectal cancer (mCRC).
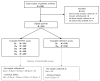
Methods: Treatment-naive patients with mCRC and measurable disease (RECIST criteria 1.1) received FOLFIRI-bevacizumab until progression or unacceptable toxicity. CTCs in peripheral blood were enumerated at D0, D14, D28, D42, and D56 (EPISPOT assay) and at D0 and D28 (CellSearch® system). Progression-free survival (PFS) and overall survival (OS) were assessed with the Kaplan-Meier method and log-rank test.
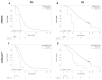
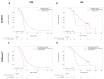
Results: With the EPISPOT assay, at least 1 viable CTC was detected in 21% (D0), 15% (D14), 12% (D28), 10% (D42), and 12% (D56) of 155 patients. PFS and OS were shorter in patients who remained positive, with viable CTCs between D0 and D28 compared with the other patients (PFS = 7.36 vs. 9.43 months, p = 0.0161 and OS = 25.99 vs. 13.83 months, p = 0.0178). The prognostic and predictive values of ≥3 CTCs (CellSearch® system) were confirmed.
Conclusions: CTC detection at D28 and the D0-D28 CTC dynamics evaluated with the EPISPOT assay were associated with outcomes and may predict response to treatment.
Keywords: CellSearch® system; EPISPOT assay; circulating tumor cells; colorectal cancer; predictive value.
Conflict of interest statement
C.A.P. has received an honorarium from Menarini. T.M. discloses research funding from ROCHE and AMGEN; an honorarium from AMGEN, SANOFI, BMS, SANDOZ, and AAA; and travel, accommodations, and expenses paid by AMGEN. The other authors declare no conflict of interest.
Figures
References
-
- Phelip J.M., Tougeron D., Léonard D., Benhaim L., Desolneux G., Dupré A., Michel P., Penna C., Tournigand C., Louvet C., et al. Metastatic colorectal cancer (mCRC): French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR) Dig. Liver Dis. 2019;51:1357–1363. doi: 10.1016/j.dld.2019.05.035. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

