n-Butylidenephthalide Modulates Autophagy to Ameliorate Neuropathological Progress of Spinocerebellar Ataxia Type 3 through mTOR Pathway
- PMID: 34199295
- PMCID: PMC8231882
- DOI: 10.3390/ijms22126339
n-Butylidenephthalide Modulates Autophagy to Ameliorate Neuropathological Progress of Spinocerebellar Ataxia Type 3 through mTOR Pathway
Abstract
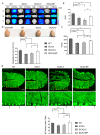
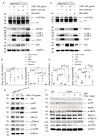
Spinocerebellar ataxia type 3 (SCA3), a hereditary and lethal neurodegenerative disease, is attributed to the abnormal accumulation of undegradable polyglutamine (polyQ), which is encoded by mutated ataxin-3 gene (ATXN3). The toxic fragments processed from mutant ATXN3 can induce neuronal death, leading to the muscular incoordination of the human body. Some treatment strategies of SCA3 are preferentially focused on depleting the abnormal aggregates, which led to the discovery of small molecule n-butylidenephthalide (n-BP). n-BP-promoted autophagy protected the loss of Purkinje cell in the cerebellum that regulates the network associated with motor functions. We report that the n-BP treatment may be effective in treating SCA3 disease. n-BP treatment led to the depletion of mutant ATXN3 with the expanded polyQ chain and the toxic fragments resulting in increased metabolic activity and alleviated atrophy of SCA3 murine cerebellum. Furthermore, n-BP treated animal and HEK-293GFP-ATXN3-84Q cell models could consistently show the depletion of aggregates through mTOR inhibition. With its unique mechanism, the two autophagic inhibitors Bafilomycin A1 and wortmannin could halt the n-BP-induced elimination of aggregates. Collectively, n-BP shows promising results for the treatment of SCA3.
Keywords: MJD; PolyQ; Purkinje cell; SCA3; atxain-3; autophagy; mTOR; toxic fragment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Anti-Excitotoxic Effects of N-Butylidenephthalide Revealed by Chemically Insulted Purkinje Progenitor Cells Derived from SCA3 iPSCs.Int J Mol Sci. 2022 Jan 26;23(3):1391. doi: 10.3390/ijms23031391. Int J Mol Sci. 2022. PMID: 35163312 Free PMC article.
-
n-Butylidenephthalide exhibits protection against neurotoxicity through regulation of tryptophan 2, 3 dioxygenase in spinocerebellar ataxia type 3.Neuropharmacology. 2017 May 1;117:434-446. doi: 10.1016/j.neuropharm.2017.02.014. Epub 2017 Feb 20. Neuropharmacology. 2017. PMID: 28223212
-
Far-infrared Radiation Improves Motor Dysfunction and Neuropathology in Spinocerebellar Ataxia Type 3 Mice.Cerebellum. 2019 Feb;18(1):22-32. doi: 10.1007/s12311-018-0936-3. Cerebellum. 2019. PMID: 29725949
-
Autophagy in Spinocerebellar Ataxia Type 3: From Pathogenesis to Therapeutics.Int J Mol Sci. 2023 Apr 17;24(8):7405. doi: 10.3390/ijms24087405. Int J Mol Sci. 2023. PMID: 37108570 Free PMC article. Review.
-
Toward therapeutic targets for SCA3: Insight into the role of Machado-Joseph disease protein ataxin-3 in misfolded proteins clearance.Prog Neurobiol. 2015 Sep;132:34-58. doi: 10.1016/j.pneurobio.2015.06.004. Epub 2015 Jun 27. Prog Neurobiol. 2015. PMID: 26123252 Review.
Cited by
-
Coenzyme Q10 Supplementation Increases Removal of the ATXN3 Polyglutamine Repeat, Reducing Cerebellar Degeneration and Improving Motor Dysfunction in Murine Spinocerebellar Ataxia Type 3.Nutrients. 2022 Aug 31;14(17):3593. doi: 10.3390/nu14173593. Nutrients. 2022. PMID: 36079853 Free PMC article.
-
Small Molecules Inducing Autophagic Degradation of Expanded Polyglutamine Protein through Interaction with Both Mutant ATXN3 and LC3.Int J Mol Sci. 2024 Oct 4;25(19):10707. doi: 10.3390/ijms251910707. Int J Mol Sci. 2024. PMID: 39409036 Free PMC article.
-
Targeting the Axl and mTOR Pathway Synergizes Immunotherapy and Chemotherapy to Butylidenephthalide in a Recurrent GBM.J Oncol. 2022 May 18;2022:3236058. doi: 10.1155/2022/3236058. eCollection 2022. J Oncol. 2022. PMID: 35646111 Free PMC article.
-
Anti-Excitotoxic Effects of N-Butylidenephthalide Revealed by Chemically Insulted Purkinje Progenitor Cells Derived from SCA3 iPSCs.Int J Mol Sci. 2022 Jan 26;23(3):1391. doi: 10.3390/ijms23031391. Int J Mol Sci. 2022. PMID: 35163312 Free PMC article.
-
Targeting PSEN1 by lnc-CYP3A43-2/miR-29b-2-5p to Reduce β Amyloid Plaque Formation and Improve Cognition Function.Int J Mol Sci. 2022 Sep 11;23(18):10554. doi: 10.3390/ijms231810554. Int J Mol Sci. 2022. PMID: 36142465 Free PMC article.
References
-
- Zhuchenko O., Bailey J., Bonnen P., Ashizawa T., Stockton D.W., Amos C., Dobyns W.B., Subramony S., Zoghbi H.Y., Lee C.C. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the α 1A-voltage-dependent calcium channel. Nat. Genet. 1997;15:62–69. doi: 10.1038/ng0197-62. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

