Inter-Metastatic Heterogeneity of Tumor Marker Expression and Microenvironment Architecture in a Preclinical Cancer Model
- PMID: 34199298
- PMCID: PMC8231937
- DOI: 10.3390/ijms22126336
Inter-Metastatic Heterogeneity of Tumor Marker Expression and Microenvironment Architecture in a Preclinical Cancer Model
Abstract
Background: Preclinical drug development studies rarely consider the impact of a candidate drug on established metastatic disease. This may explain why agents that are successful in subcutaneous and even orthotopic preclinical models often fail to demonstrate efficacy in clinical trials. It is reasonable to anticipate that sites of metastasis will be phenotypically unique, as each tumor will have evolved heterogeneously with respect to gene expression as well as the associated phenotypic outcome of that expression. The objective for the studies described here was to gain an understanding of the tumor heterogeneity that exists in established metastatic disease and use this information to define a preclinical model that is more predictive of treatment outcome when testing novel drug candidates clinically.
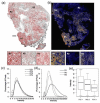
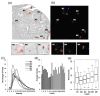
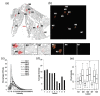
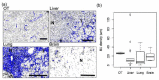
Methods: Female NCr nude mice were inoculated with fluorescent (mKate), Her2/neu-positive human breast cancer cells (JIMT-mKate), either in the mammary fat pad (orthotopic; OT) to replicate a primary tumor, or directly into the left ventricle (intracardiac; IC), where cells eventually localize in multiple sites to create a model of established metastasis. Tumor development was monitored by in vivo fluorescence imaging (IVFI). Subsequently, animals were sacrificed, and tumor tissues were isolated and imaged ex vivo. Tumors within organ tissues were further analyzed via multiplex immunohistochemistry (mIHC) for Her2/neu expression, blood vessels (CD31), as well as a nuclear marker (Hoechst) and fluorescence (mKate) expressed by the tumor cells.
Results: Following IC injection, JIMT-1mKate cells consistently formed tumors in the lung, liver, brain, kidney, ovaries, and adrenal glands. Disseminated tumors were highly variable when assessing vessel density (CD31) and tumor marker expression (mkate, Her2/neu). Interestingly, tumors which developed within an organ did not adopt a vessel microarchitecture that mimicked the organ where growth occurred, nor did the vessel microarchitecture appear comparable to the primary tumor. Rather, metastatic lesions showed considerable variability, suggesting that each secondary tumor is a distinct disease entity from a microenvironmental perspective.
Conclusions: The data indicate that more phenotypic heterogeneity in the tumor microenvironment exists in models of metastatic disease than has been previously appreciated, and this heterogeneity may better reflect the metastatic cancer in patients typically enrolled in early-stage Phase I/II clinical trials. Similar to the suggestion of others in the past, the use of models of established metastasis preclinically should be required as part of the anticancer drug candidate development process, and this may be particularly important for targeted therapeutics and/or nanotherapeutics.
Keywords: chemoresistance; drug development; inter-metastatic heterogeneity; metastasis; multiplex immunohistochemistry; preclinical studies; translational medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Takimoto C.H. Why Drugs Fail: Of Mice and Men Revisited. Clin. Cancer Res. 2001;7:229–230. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

