The Role of Biodegradable Poly-(L-lactide)-Based Polymers in Blood Cell Activation and Platelet-Monocyte Interaction
- PMID: 34199303
- PMCID: PMC8231768
- DOI: 10.3390/ijms22126340
The Role of Biodegradable Poly-(L-lactide)-Based Polymers in Blood Cell Activation and Platelet-Monocyte Interaction
Abstract
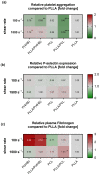
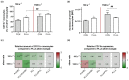
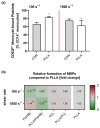
The main purpose of new stent technologies is to overcome unfavorable material-related incompatibilities by producing bio- and hemo-compatible polymers with anti-inflammatory and anti-thrombogenic properties. In this context, wettability is an important surface property, which has a major impact on the biological response of blood cells. However, the influence of local hemodynamic changes also influences blood cell activation. Therefore, we investigated biodegradable polymers with different wettability to identify possible aspects for a better prediction of blood compatibility. We applied shear rates of 100 s-1 and 1500 s-1 and assessed platelet and monocyte activation as well as the formation of CD62P+ monocyte-bound platelets via flow cytometry. Aggregation of circulating platelets induced by collagen was assessed by light transmission aggregometry. Via live cell imaging, leukocytes were tracked on biomaterial surfaces to assess their average velocity. Monocyte adhesion on biomaterials was determined by fluorescence microscopy. In response to low shear rates of 100 s-1, activation of circulating platelets and monocytes as well as the formation of CD62P+ monocyte-bound platelets corresponded to the wettability of the underlying material with the most favorable conditions on more hydrophilic surfaces. Under high shear rates, however, blood compatibility cannot only be predicted by the concept of wettability. We assume that the mechanisms of blood cell-polymer interactions do not allow for a rule-of-thumb prediction of the blood compatibility of a material, which makes extensive in vitro testing mandatory.
Keywords: leukocyte activation; platelet activation; platelet–monocyte aggregates; poly-(L-lactide); shear stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
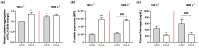



Similar articles
-
New stent surface materials: the impact of polymer-dependent interactions of human endothelial cells, smooth muscle cells, and platelets.Acta Biomater. 2014 Feb;10(2):688-700. doi: 10.1016/j.actbio.2013.10.015. Epub 2013 Oct 19. Acta Biomater. 2014. PMID: 24148751
-
The GPIIb/IIIa antagonist eptifibatide markedly potentiates platelet-leukocyte interaction and tissue factor expression following platelet activation in whole blood in vitro.Platelets. 2002 Nov;13(7):401-6. doi: 10.1080/0953710021000024367. Platelets. 2002. PMID: 12487787
-
Preferential binding of platelets to monocytes over neutrophils under flow.Biochem Biophys Res Commun. 2005 Apr 1;329(1):345-55. doi: 10.1016/j.bbrc.2005.01.146. Biochem Biophys Res Commun. 2005. PMID: 15721313
-
Aspirin and P2Y12 Inhibitors in platelet-mediated activation of neutrophils and monocytes.Thromb Haemost. 2015 Aug 31;114(3):478-89. doi: 10.1160/TH14-11-0943. Epub 2015 Apr 23. Thromb Haemost. 2015. PMID: 25904241 Review.
-
Platelet-leukocyte aggregates and derived microparticles in inflammation, vascular remodelling and thrombosis.Front Biosci. 2006 Jan 1;11:830-7. doi: 10.2741/1840. Front Biosci. 2006. PMID: 16146774 Review.
Cited by
-
Conditioned plasma promotes full-thickness skin defect healing in a rat model.Regen Ther. 2025 Aug 18;30:629-640. doi: 10.1016/j.reth.2025.08.003. eCollection 2025 Dec. Regen Ther. 2025. PMID: 40896180 Free PMC article.
-
Predicting the In Vivo Performance of Cardiovascular Biomaterials: Current Approaches In Vitro Evaluation of Blood-Biomaterial Interactions.Int J Mol Sci. 2021 Oct 21;22(21):11390. doi: 10.3390/ijms222111390. Int J Mol Sci. 2021. PMID: 34768821 Free PMC article. Review.
References
-
- Fischer M., Maitz M.F., Werner C. Coatings for biomaterials to improve hemocompatibility. In: Siedlecki C.A., editor. Hemocompatibility of Biomaterials for Clinical Applications. Woodhead Publishing; Sawston, UK: 2018. pp. 163–190.
-
- Virmani R., Guagliumi G., Farb A., Musumeci G., Grieco N., Motta T., Mihalcsik L., Tespili M., Valsecchi O., Kolodgie F.D. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent should we be cautious? Circulation. 2004;109:701–705. doi: 10.1161/01.CIR.0000116202.41966.D4. - DOI - PubMed
-
- Mann G.S., Singh L.P., Kumar P., Singh S., Prakash C. On briefing the surface modifications of polylactic acid: A scope for betterment of biomedical structures. J. Thermoplast. Compos. Mater. 2019 doi: 10.1177/0892705719856052. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

