The In Vitro α-Glucosidase Inhibition Activity of Various Solvent Fractions of Tamarix dioica and 1H-NMR Based Metabolite Identification and Molecular Docking Analysis
- PMID: 34199333
- PMCID: PMC8227178
- DOI: 10.3390/plants10061128
The In Vitro α-Glucosidase Inhibition Activity of Various Solvent Fractions of Tamarix dioica and 1H-NMR Based Metabolite Identification and Molecular Docking Analysis
Abstract
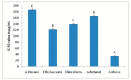
The Tamarix dioica (T. dioica) is widely used medicinal plant to cure many chronic ailments. T. dioica is being used to manage diabetes mellitus in traditional medicinal system; however, very little scientific evidence is available on this plant in this context. The current study involves the fractionation of crude methanolic extract of T. dioica using n-hexane, ethyl acetate, chloroform, and n-butanol. The screening for antioxidant activity using 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay was carried out. The in vitro antidiabetic potential was assessed by measuring α-glucosidase inhibition. Total phenolic and flavonoid contents were also determined for each fraction. The metabolites were identified using highly sensitive and emerging 1H-NMR technique. The results revealed the ethyl acetate fraction as the most potent with DPPH scavenging activity of 84.44 ± 0.21% and α-glucosidase inhibition with IC50 value of 122.81 ± 2.05 µg/mL. The total phenolic and flavonoid content values of 205.45 ± 1.36 mg gallic acid equivalent per gram dried extract and 156.85 ± 1.33 mg quercetin equivalent per gram dried extract were obtained for ethyl acetate fraction. The bucketing of 1H-NMR spectra identified 22 metabolites including some pharmacologically important like tamarixetin, tamaridone, quercetin, rutin, apigenin, catechin, kaempferol, myricetin and isorhamnetin. Leucine, lysine, glutamic acid, aspartic acid, serine, and tyrosine were the major amino acids identified in ethyl acetate fraction. The molecular docking analysis provided significant information on the binding affinity among secondary metabolites and α-glucosidase. These metabolites were most probably responsible for the antioxidant activity and α-glucosidase inhibitory potential of ethyl acetate fraction. The study ascertained the ethnomedicinal use of T. dioica to manage diabetes mellitus and may be a helpful lead towards naturopathic mode for anti-hyperglycemia.
Keywords: 1H-NMR; Tamarix dioica; antioxidant; docking; metabolites; α-glucosidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Bughio S.H., Samejo M.Q., Memon S., Bano S., Mughal M.A., Memon A.A. Chemical composition of the essential oils from Tamarix dioica and determination of its antibacterial activity. Int. J. Food Prop. 2017;20:S2660–S2667. doi: 10.1080/10942912.2017.1387138. - DOI
-
- Khan S., Khan G.M., Mehsud S., Rahman A., Khan F. Antifungal activity of Tamarix dioica—An in vitro study. Gomal J. Med. Sci. 2004;2:40–42.
-
- Samejo M.Q., Sumbul A., Shah S., Memon S.B., Chundrigar S. Phytochemical screening of Tamarix dioica Roxb. ex Roch. J. Pharm. Res. 2013;7:181–183. doi: 10.1016/j.jopr.2013.02.017. - DOI
-
- Imtiaz S.M., Aleem A., Saqib F., Ormenisan A.N., Elena Neculau A., Anastasiu C.V. The potential involvement of an ATP-dependent potassium channel-opening mechanism in the smooth muscle relaxant properties of Tamarix dioica Roxb. Biomolecules. 2019;9:722. doi: 10.3390/biom9110722. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

