In Situ Decarboxylation-Pressurized Hot Water Extraction for Selective Extraction of Cannabinoids from Cannabis sativa. Chemometric Approach
- PMID: 34199346
- PMCID: PMC8199533
- DOI: 10.3390/molecules26113343
In Situ Decarboxylation-Pressurized Hot Water Extraction for Selective Extraction of Cannabinoids from Cannabis sativa. Chemometric Approach
Abstract
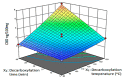
Isolation of the therapeutic cannabinoid compounds from Cannabis Sativa L. (C. Sativa) is important for the development of cannabis-based pharmaceuticals for cancer treatment, among other ailments. The main pharmacological cannabinoids are THC and CBD. However, THC also induces undesirable psychoactive effects. The decarboxylation process converts the naturally occurring acidic forms of cannabinoids, such as cannabidiolic acid (CBDA) and tetrahydrocannabinolic acid (THCA), to their more active neutral forms, known as cannabidiol (CBD) and tetrahydrocannabinol (THC). The purpose of this study was to selectively extract cannabinoids using a novel in situ decarboxylation pressurized hot water extraction (PHWE) system. The decarboxylation step was evaluated at different temperature (80-150 °C) and time (5-60 min) settings to obtain the optimal conditions for the decarboxylation-PHWE system using response surface methodology (RSM). The system was optimized to produce cannabis extracts with high CBD content, while suppressing the THC and CBN content. The identification and quantification of cannabinoid compounds were determined using UHPLC-MS/MS with external calibration. As a result, the RSM has shown good predictive capability with a p-value < 0.05, and the chosen parameters revealed to have a significant effect on the CBD, CBN and THC content. The optimal decarboxylation conditions for an extract richer in CBD than THC were set at 149.9 °C and 42 min as decarboxylation temperature and decarboxylation time, respectively. The extraction recoveries ranged between 96.56 and 103.42%, 95.22 and 99.95%, 99.62 and 99.81% for CBD, CBN and THC, respectively.
Keywords: cannabinoid compounds; decarboxylation; green extraction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Selective Extraction of Cannabinoid Compounds from Cannabis Seed Using Pressurized Hot Water Extraction.Molecules. 2020 Mar 15;25(6):1335. doi: 10.3390/molecules25061335. Molecules. 2020. PMID: 32183432 Free PMC article.
-
Evaluation of cannabinoids concentration and stability in standardized preparations of cannabis tea and cannabis oil by ultra-high performance liquid chromatography tandem mass spectrometry.Clin Chem Lab Med. 2017 Aug 28;55(10):1555-1563. doi: 10.1515/cclm-2016-1060. Clin Chem Lab Med. 2017. PMID: 28207408
-
Incomplete Decarboxylation of Acidic Cannabinoids in GC-MS Leads to Underestimation of the Total Cannabinoid Content in Cannabis Oils Without Derivatization.Pharmaceutics. 2025 Mar 5;17(3):334. doi: 10.3390/pharmaceutics17030334. Pharmaceutics. 2025. PMID: 40142998 Free PMC article.
-
Phytocannabinoids - An Overview of the Analytical Methodologies for Detection and Quantification of Therapeutically and Recreationally Relevant Cannabis Compounds.Crit Rev Anal Chem. 2023;53(1):211-231. doi: 10.1080/10408347.2021.1949694. Epub 2021 Jul 30. Crit Rev Anal Chem. 2023. PMID: 34328047 Review.
-
Cannabis sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer.Biomed Res Int. 2018 Dec 4;2018:1691428. doi: 10.1155/2018/1691428. eCollection 2018. Biomed Res Int. 2018. PMID: 30627539 Free PMC article. Review.
Cited by
-
Analytical Techniques for Phytocannabinoid Profiling of Cannabis and Cannabis-Based Products-A Comprehensive Review.Molecules. 2022 Feb 1;27(3):975. doi: 10.3390/molecules27030975. Molecules. 2022. PMID: 35164240 Free PMC article. Review.
-
Postharvest Operations of Cannabis and Their Effect on Cannabinoid Content: A Review.Bioengineering (Basel). 2022 Aug 3;9(8):364. doi: 10.3390/bioengineering9080364. Bioengineering (Basel). 2022. PMID: 36004888 Free PMC article. Review.
-
Simple Extraction of Cannabinoids from Female Inflorescences of Hemp (Cannabis sativa L.).Molecules. 2022 Sep 10;27(18):5868. doi: 10.3390/molecules27185868. Molecules. 2022. PMID: 36144607 Free PMC article.
References
-
- Aladic K., Jarni K., Barbir T., Vidovic S., Vladic J., Bilic M., Jokic S. Supercritical CO2 extraction of hemp (Cannabis sativa L.) seed oil. Ind. Crops Prod. 2015;76:472–478. doi: 10.1016/j.indcrop.2015.07.016. - DOI
-
- Rovetto L.J., Aieta N.V. Supercritical carbon dioxide extraction of cannabinoids from Cannabis sativa L. J. Supercrit. Fluids. 2017;129:16–27. doi: 10.1016/j.supflu.2017.03.014. - DOI
-
- Devi V., Khanam S. Study of ω-6 linoleic and ω-3 α-linolenic acids of hemp (Cannabis sativa) seed oil extracted by supercritical CO2 extraction: CCD optimization. J. Environ. Chem. Eng. 2019;7:102818. doi: 10.1016/j.jece.2018.102818. - DOI
-
- Bernstein N., Gorelick J., Koch S. Interplay between chemistry and morphology in medical cannabis (Cannabis sativa L.) Ind. Crops Prod. 2019;129:185–194. doi: 10.1016/j.indcrop.2018.11.039. - DOI
-
- Moreno T., Montanes F., Tallon S.J., King J. Extraction of cannabinoids from hemp (Cannabis sativa L.) using high pressure solvents: An overview of different processing options. J. Supercrit. Fluids. 2020;161:104850. doi: 10.1016/j.supflu.2020.104850. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials