Multifactorial Pathogenic Processes of Retinal Ganglion Cell Degeneration in Glaucoma towards Multi-Target Strategies for Broader Treatment Effects
- PMID: 34199494
- PMCID: PMC8228726
- DOI: 10.3390/cells10061372
Multifactorial Pathogenic Processes of Retinal Ganglion Cell Degeneration in Glaucoma towards Multi-Target Strategies for Broader Treatment Effects
Abstract
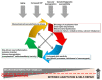
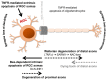
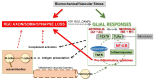
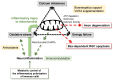
Glaucoma is a chronic neurodegenerative disease characterized by apoptosis of retinal ganglion cell (RGC) somas, degeneration of axons, and loss of synapses at dendrites and axon terminals. Glaucomatous neurodegeneration encompasses multiple triggers, multiple cell types, and multiple molecular pathways through the etiological paths with biomechanical, vascular, metabolic, oxidative, and inflammatory components. As much as intrinsic responses of RGCs themselves, divergent responses and intricate interactions of the surrounding glia also play decisive roles for the cell fate. Seen from a broad perspective, multitarget treatment strategies have a compelling pathophysiological basis to more efficiently manipulate multiple pathogenic processes at multiple injury sites in such a multifactorial neurodegenerative disease. Despite distinct molecular programs for somatic and axonal degeneration, mitochondrial dysfunction and glia-driven neuroinflammation present interdependent processes with widespread impacts in the glaucomatous retina and optic nerve. Since dysfunctional mitochondria stimulate inflammatory responses and proinflammatory mediators impair mitochondria, mitochondrial restoration may be immunomodulatory, while anti-inflammatory treatments protect mitochondria. Manipulation of these converging routes may thus allow a unified treatment strategy to protect RGC axons, somas, and synapses. This review presents an overview of recent research advancements with emphasis on potential treatment targets to achieve the best treatment efficacy to preserve visual function in glaucoma.
Keywords: glaucoma; glia; immunomodulation; neurodegeneration; neuroinflammation; neuroprotection; retinal ganglion cell.
Conflict of interest statement
The author declares no conflict of interest.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

