Sialic Acids as Receptors for Pathogens
- PMID: 34199560
- PMCID: PMC8227644
- DOI: 10.3390/biom11060831
Sialic Acids as Receptors for Pathogens
Abstract
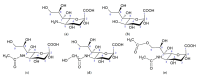
Carbohydrates have long been known to mediate intracellular interactions, whether within one organism or between different organisms. Sialic acids (Sias) are carbohydrates that usually occupy the terminal positions in longer carbohydrate chains, which makes them common recognition targets mediating these interactions. In this review, we summarize the knowledge about animal disease-causing agents such as viruses, bacteria and protozoa (including the malaria parasite Plasmodium falciparum) in which Sias play a role in infection biology. While Sias may promote binding of, e.g., influenza viruses and SV40, they act as decoys for betacoronaviruses. The presence of two common forms of Sias, Neu5Ac and Neu5Gc, is species-specific, and in humans, the enzyme converting Neu5Ac to Neu5Gc (CMAH, CMP-Neu5Ac hydroxylase) is lost, most likely due to adaptation to pathogen regimes; we discuss the research about the influence of malaria on this trait. In addition, we present data suggesting the CMAH gene was probably present in the ancestor of animals, shedding light on its glycobiology. We predict that a better understanding of the role of Sias in disease vectors would lead to more effective clinical interventions.
Keywords: CMAH gene; phylogenetic tree; receptors for pathogens; sialic acids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

