Comparison of Oncologic Outcomes of Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin (ddMVAC) with Gemcitabine and Cisplatin (GC) as Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: Systematic Review and Meta-Analysis
- PMID: 34199565
- PMCID: PMC8199668
- DOI: 10.3390/cancers13112770
Comparison of Oncologic Outcomes of Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin (ddMVAC) with Gemcitabine and Cisplatin (GC) as Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: Systematic Review and Meta-Analysis
Abstract
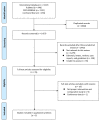
Platinum-based neoadjuvant chemotherapy (NAC) is widely used for treating muscle-invasive bladder cancer (MIBC). A systematic review was performed following PRISMA guidelines. PubMed, Embase, and the Cochrane Library were searched up to December 2020. We conducted a meta-analysis to compare the oncologic outcomes of ddMVAC (dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin) and GC (gemcitabine and cisplatin), which are the most widely used NAC regimens. Endpoints included pathologic complete response (pCR), pathologic downstaging (pDS), overall survival (OS), and cancer-specific survival (CSS). Five studies, with a total of 1206 patients, were included for meta-analysis. pCR was observed in 35.2% of the ddMVAC arm and in 25.1% of the GC arm, and pCR was significantly higher in ddMVAC than in GC (odds ratio (OR), 1.45; 95% confidence interval (CI), 1.11-1.89; p = 0.006). There was no significant difference in pDS (OR, 1.37; CI, 0.84-2.21; p = 0.20). OS was significantly higher in ddMVAC than in GC (hazard ratio, 2.16; CI, 1.42-3.29; p = 0.0004). Only one study reported CSS outcomes. The results of this analysis indicate that ddMVAC is superior to GC in terms of pCR and OS, suggesting that ddMVAC is more effective than GC in NAC for MIBC. However, this should be interpreted with caution because of the inherent limitations of retrospective studies.
Keywords: bladder cancer; cisplatin; dose-dense MVAC; gemcitabine; neoadjuvant chemotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bamias A., Dafni U., Karadimou A., Timotheadou E., Aravantinos G., Psyrri A., Xanthakis I., Tsiatas M., Koutoulidis V., Constantinidis C., et al. Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: A Hellenic Cooperative Oncology Group study (HE 16/03) Ann. Oncol. Off. J. Eur. Soc. Med Oncol. 2013;24:1011–1017. doi: 10.1093/annonc/mds583. - DOI - PubMed
-
- Mari A., Campi R., Tellini R., Gandaglia G., Albisinni S., Abufaraj M., Hatzichristodoulou G., Montorsi F., van Velthoven R., Carini M., et al. Patterns and predictors of recurrence after open radical cystectomy for bladder cancer: A comprehensive review of the literature. World J. Urol. 2018;36:157–170. doi: 10.1007/s00345-017-2115-4. - DOI - PMC - PubMed
-
- Shariat S.F., Karakiewicz P.I., Palapattu G.S., Lotan Y., Rogers C.G., Amiel G.E., Vazina A., Gupta A., Bastian P.J., Sagalowsky A.I., et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: A contemporary series from the bladder cancer research consortium. J. Urol. 2006;176:2414–2422. doi: 10.1016/j.juro.2006.08.004. - DOI - PubMed
-
- Stein J.P., Lieskovsky G., Cote R., Groshen S., Feng A.-C., Boyd S., Skinner E., Bochner B., Thangathurai D., Mikhail M., et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1054 patients. J. Clin. Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

