Functional Characterization of the Dopaminergic Psychostimulant Sydnocarb as an Allosteric Modulator of the Human Dopamine Transporter
- PMID: 34199621
- PMCID: PMC8227285
- DOI: 10.3390/biomedicines9060634
Functional Characterization of the Dopaminergic Psychostimulant Sydnocarb as an Allosteric Modulator of the Human Dopamine Transporter
Abstract
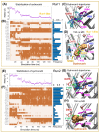
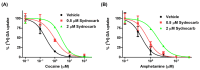
The dopamine transporter (DAT) serves a critical role in controlling dopamine (DA)-mediated neurotransmission by regulating the clearance of DA from the synapse and extrasynaptic regions and thereby modulating DA action at postsynaptic DA receptors. Major drugs of abuse such as amphetamine and cocaine interact with DATs to alter their actions resulting in an enhancement in extracellular DA concentrations. We previously identified a novel allosteric site in the DAT and the related human serotonin transporter that lies outside the central orthosteric substrate- and cocaine-binding pocket. Here, we demonstrate that the dopaminergic psychostimulant sydnocarb is a ligand of this novel allosteric site. We identified the molecular determinants of the interaction between sydnocarb and DAT at the allosteric site using molecular dynamics simulations. Biochemical-substituted cysteine scanning accessibility experiments have supported the computational predictions by demonstrating the occurrence of specific interactions between sydnocarb and amino acids within the allosteric site. Functional dopamine uptake studies have further shown that sydnocarb is a noncompetitive inhibitor of DAT in accord with the involvement of a site different from the orthosteric site in binding this psychostimulant. Finally, DA uptake studies also demonstrate that sydnocarb affects the interaction of DAT with both cocaine and amphetamine. In summary, these studies further strengthen the prospect that allosteric modulation of DAT activity could have therapeutic potential.
Keywords: allosteric modulation; dopamine transporter; transport activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Davidson C. Developing Treatments for Stimulant Abuse: A Brief Overview. East Asian Arch. Psychiatry. 2016;26:52–59. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

