Exploring the Physiological Role of Transthyretin in Glucose Metabolism in the Liver
- PMID: 34199897
- PMCID: PMC8200108
- DOI: 10.3390/ijms22116073
Exploring the Physiological Role of Transthyretin in Glucose Metabolism in the Liver
Abstract
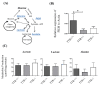
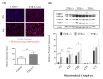
Transthyretin (TTR), a 55 kDa evolutionarily conserved protein, presents altered levels in several conditions, including malnutrition, inflammation, diabetes, and Alzheimer's Disease. It has been shown that TTR is involved in several functions, such as insulin release from pancreatic β-cells, recovery of blood glucose and glucagon levels of the islets of Langerhans, food intake, and body weight. Here, the role of TTR in hepatic glucose metabolism was explored by studying the levels of glucose in mice with different TTR genetic backgrounds, namely with two copies of the TTR gene, TTR+/+; with only one copy, TTR+/-; and without TTR, TTR-/-. Results showed that TTR haploinsufficiency (TTR+/-) leads to higher glucose in both plasma and in primary hepatocyte culture media and lower expression of the influx glucose transporters, GLUT1, GLUT3, and GLUT4. Further, we showed that TTR haploinsufficiency decreases pyruvate kinase M type (PKM) levels in mice livers, by qRT-PCR, but it does not affect the hepatic production of the studied metabolites, as determined by 1H NMR. Finally, we demonstrated that TTR increases mitochondrial density in HepG2 cells and that TTR insufficiency triggers a higher degree of oxidative phosphorylation in the liver. Altogether, these results indicate that TTR contributes to the homeostasis of glucose by regulating the levels of glucose transporters and PKM enzyme and by protecting against mitochondrial oxidative stress.
Keywords: glucose metabolism; glucose transporters; liver; mitochondria; transthyretin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

