Silica Containing Composite Anion Exchange Membranes by Sol-Gel Synthesis: A Short Review
- PMID: 34200025
- PMCID: PMC8200225
- DOI: 10.3390/polym13111874
Silica Containing Composite Anion Exchange Membranes by Sol-Gel Synthesis: A Short Review
Abstract
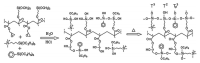
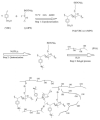
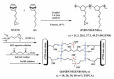
This short review summarizes the literature on composite anion exchange membranes (AEM) containing an organo-silica network formed by sol-gel chemistry. The article covers AEM for diffusion dialysis (DD), for electrochemical energy technologies including fuel cells and redox flow batteries, and for electrodialysis. By applying a vast variety of organically modified silica compounds (ORMOSIL), many composite AEM reported in the last 15 years are based on poly (vinylalcohol) (PVA) or poly (2,6-dimethyl-1,4-phenylene oxide) (PPO) used as polymer matrix. The most stringent requirements are high permselectivity and water flux for DD membranes, while high ionic conductivity is essential for electrochemical applications. Furthermore, the alkaline stability of AEM for fuel cell applications remains a challenging problem that is not yet solved. Possible future topics of investigation on composite AEM containing an organo-silica network are also discussed.
Keywords: diffusion dialysis; electrodialysis; fuel cells; ionomers; ormosils; redox flow batteries.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Merle G., Wessling M., Nijmeijer K. Anion exchange membranes for alkaline fuel cells: A review. J. Membr. Sci. 2011;377:1–35. doi: 10.1016/j.memsci.2011.04.043. - DOI
-
- Hickner M.A., Herring A.M., Coughlin E.B. Anion exchange membranes: Current status and moving forward. J. Polym. Sci. Part B Polym. Phys. 2013;51:1727–1735. doi: 10.1002/polb.23395. - DOI
-
- Knauth P., Pasquini L., Narducci R., Sgreccia E., Becerra-Arciniegas R.-A., Di Vona M.L. Effective ion mobility in anion exchange ionomers: Relations with hydration, porosity, tortuosity, and percolation. J. Membr. Sci. 2021;617:118622. doi: 10.1016/j.memsci.2020.118622. - DOI
-
- Kreuer K.-D. Ion conducting membranes for fuel cells and other electrochemical devices. Chem. Mater. 2014;26:361–380. doi: 10.1021/cm402742u. - DOI
-
- Maurya S., Shin S.-H., Kim Y., Moon S.-H. A review on recent developments of anion exchange membranes for fuel cells and redox flow batteries. RSC Adv. 2015;5:37206–37230. doi: 10.1039/C5RA04741B. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

