Cystic Fibrosis Sputum Impairs the Ability of Neutrophils to Kill Staphylococcus aureus
- PMID: 34200034
- PMCID: PMC8229215
- DOI: 10.3390/pathogens10060703
Cystic Fibrosis Sputum Impairs the Ability of Neutrophils to Kill Staphylococcus aureus
Abstract
Cystic fibrosis (CF) airway disease is characterized by chronic microbial infections and infiltration of inflammatory polymorphonuclear (PMN) granulocytes. Staphylococcus aureus (S. aureus) is a major lung pathogen in CF that persists despite the presence of PMNs and has been associated with CF lung function decline. While PMNs represent the main mechanism of the immune system to kill S. aureus, it remains largely unknown why PMNs fail to eliminate S. aureus in CF. The goal of this study was to observe how the CF airway environment affects S. aureus killing by PMNs. PMNs were isolated from the blood of healthy volunteers and CF patients. Clinical isolates of S. aureus were obtained from the airways of CF patients. The results show that PMNs from healthy volunteers were able to kill all CF isolates and laboratory strains of S. aureus tested in vitro. The extent of killing varied among strains. When PMNs were pretreated with supernatants of CF sputum, S. aureus killing was significantly inhibited suggesting that the CF airway environment compromises PMN antibacterial functions. CF blood PMNs were capable of killing S. aureus. Although bacterial killing was inhibited with CF sputum, PMN binding and phagocytosis of S. aureus was not diminished. The S. aureus-induced respiratory burst and neutrophil extracellular trap release from PMNs also remained uninhibited by CF sputum. In summary, our data demonstrate that the CF airway environment limits killing of S. aureus by PMNs and provides a new in vitro experimental model to study this phenomenon and its mechanism.
Keywords: PMN; Staphylococcus aureus; cystic fibrosis; killing; neutrophil extracellular traps; respiratory burst; sputum.
Conflict of interest statement
The authors have no conflict of interest related to this work.
Figures
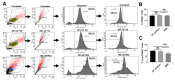
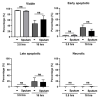
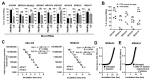
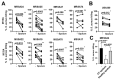
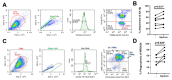
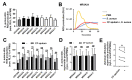
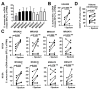
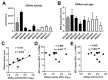
References
-
- Ahlgren H.G., Benedetti A., Landry J.S., Bernier J., Matouk E., Radzioch D., Lands L.C., Rousseau S., Nguyen D. Clinical outcomes associated with Staphylococcus aureus and Pseudomonas aeruginosa airway infections in adult cystic fibrosis patients. BMC Pulm. Med. 2015;15:67. doi: 10.1186/s12890-015-0062-7. - DOI - PMC - PubMed
-
- Cystic Fibrosis Foundation Patient Registry . 2017 Annual Data Report. Cystic Fibrosis Foundation Patient Registry; Bethesda, MD, USA: 2018.
-
- Muhlebach M.S., Zorn B.T., Esther C.R., Hatch J.E., Murray C.P., Turkovic L., Ranganathan S.C., Boucher R.C., Stick S.M., Wolfgang M.C. Initial acquisition and succession of the cystic fibrosis lung microbiome is associated with disease progression in infants and preschool children. PLoS Pathog. 2018;14:e1006798. doi: 10.1371/journal.ppat.1006798. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

