Adrenergic and Glucocorticoid Receptors in the Pulmonary Health Effects of Air Pollution
- PMID: 34200050
- PMCID: PMC8226814
- DOI: 10.3390/toxics9060132
Adrenergic and Glucocorticoid Receptors in the Pulmonary Health Effects of Air Pollution
Abstract
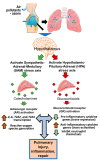
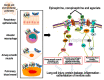
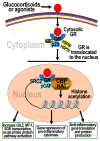
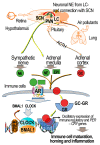
Adrenergic receptors (ARs) and glucocorticoid receptors (GRs) are activated by circulating catecholamines and glucocorticoids, respectively. These receptors regulate the homeostasis of physiological processes with specificity via multiple receptor subtypes, wide tissue-specific distribution, and interactions with other receptors and signaling processes. Based on their physiological roles, ARs and GRs are widely manipulated therapeutically for chronic diseases. Although these receptors play key roles in inflammatory and cellular homeostatic processes, little research has addressed their involvement in the health effects of air pollution. We have recently demonstrated that ozone, a prototypic air pollutant, mediates pulmonary and systemic effects through the activation of these receptors. A single exposure to ozone induces the sympathetic-adrenal-medullary and hypothalamic-pituitary-adrenal axes, resulting in the release of epinephrine and corticosterone into the circulation. These hormones act as ligands for ARs and GRs. The roles of beta AR (βARs) and GRs in ozone-induced pulmonary injury and inflammation were confirmed in a number of studies using interventional approaches. Accordingly, the activation status of ARs and GRs is critical in mediating the health effects of inhaled irritants. In this paper, we review the cellular distribution and functions of ARs and GRs, their lung-specific localization, and their involvement in ozone-induced health effects, in order to capture attention for future research.
Keywords: adrenergic receptors; air pollution; glucocorticoid receptors; inflammation; lung injury; ozone.
Conflict of interest statement
Authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
Adrenergic and glucocorticoid receptor antagonists reduce ozone-induced lung injury and inflammation.Toxicol Appl Pharmacol. 2018 Jan 15;339:161-171. doi: 10.1016/j.taap.2017.12.006. Epub 2017 Dec 13. Toxicol Appl Pharmacol. 2018. PMID: 29247675 Free PMC article.
-
Neuroendocrine Regulation of Air Pollution Health Effects: Emerging Insights.Toxicol Sci. 2018 Jul 1;164(1):9-20. doi: 10.1093/toxsci/kfy129. Toxicol Sci. 2018. PMID: 29846720 Free PMC article. Review.
-
Independent roles of beta-adrenergic and glucocorticoid receptors in systemic and pulmonary effects of ozone.Inhal Toxicol. 2020 Mar;32(4):155-169. doi: 10.1080/08958378.2020.1759736. Epub 2020 May 4. Inhal Toxicol. 2020. PMID: 32366144 Free PMC article.
-
Beta-2 Adrenergic and Glucocorticoid Receptor Agonists Modulate Ozone-Induced Pulmonary Protein Leakage and Inflammation in Healthy and Adrenalectomized Rats.Toxicol Sci. 2018 Dec 1;166(2):288-305. doi: 10.1093/toxsci/kfy198. Toxicol Sci. 2018. PMID: 30379318 Free PMC article.
-
Stretching the stress boundary: Linking air pollution health effects to a neurohormonal stress response.Biochim Biophys Acta. 2016 Dec;1860(12):2880-90. doi: 10.1016/j.bbagen.2016.05.010. Epub 2016 May 8. Biochim Biophys Acta. 2016. PMID: 27166979 Review.
Cited by
-
Differential transcriptomic alterations in nasal versus lung tissue of acrolein-exposed rats.Front Toxicol. 2023 Nov 27;5:1280230. doi: 10.3389/ftox.2023.1280230. eCollection 2023. Front Toxicol. 2023. PMID: 38090360 Free PMC article.
-
Serum metabolome and liver transcriptome reveal acrolein inhalation-induced sex-specific homeostatic dysfunction.Sci Rep. 2023 Dec 1;13(1):21179. doi: 10.1038/s41598-023-48413-w. Sci Rep. 2023. PMID: 38040807 Free PMC article.
-
Relationships between Long-Term Ozone Exposure and Allergic Rhinitis and Bronchitic Symptoms in Chinese Children.Toxics. 2021 Sep 14;9(9):221. doi: 10.3390/toxics9090221. Toxics. 2021. PMID: 34564372 Free PMC article.
-
Air Pollutant impacts on the brain and neuroendocrine system with implications for peripheral organs: a perspective.Inhal Toxicol. 2023 Mar-Apr;35(3-4):109-126. doi: 10.1080/08958378.2023.2172486. Epub 2023 Feb 7. Inhal Toxicol. 2023. PMID: 36749208 Free PMC article. Review.
-
Effects of Ambient O3 on Respiratory Mortality, Especially the Combined Effects of PM2.5 and O3.Toxics. 2023 Oct 30;11(11):892. doi: 10.3390/toxics11110892. Toxics. 2023. PMID: 37999544 Free PMC article.
References
-
- Brinchmann B.C., Le Ferrec E., Podechard N., Lagadic-Gossmann M., Holme J.A., Øvrevik J. Organic chemicals from diesel exhaust particles affects intracellular calcium, inflammation and β-adrenoceptors in endothelial cells. Toxicol. Lett. 2019;302:18–27. doi: 10.1016/j.toxlet.2018.11.009. - DOI - PubMed
-
- Henriquez A.R., Snow S.J., Schladweiler M.C., Miller C.N., Dye J., Ledbetter A.D., Richards J., Hargrove M.M., Williams W.C., Kodavanti U.P. Beta-2 Adrenergic and Glucocorticoid Receptor Agonists Modulate Ozone-Induced Pulmonary Protein Leakage and Inflammation in Healthy and Adrenalectomized Rats. Toxicol. Sci. 2018;166:288–305. doi: 10.1093/toxsci/kfy198. - DOI - PMC - PubMed
-
- Henriquez A.R., Snow S.J., Schladweiler M.C., Miller C.N., Dye J.A., Ledbetter A.D., Richards J.E., Mauge-Lewis K., McGee M.A., Kodavanti U.P. Adrenergic and glucocorticoid receptor antagonists reduce ozone-induced lung injury and inflammation. Toxicol. Appl. Pharmacol. 2018;339:161–171. doi: 10.1016/j.taap.2017.12.006. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

