New Oxaliplatin-Pyrophosphato Analogs with Improved In Vitro Cytotoxicity
- PMID: 34200051
- PMCID: PMC8200237
- DOI: 10.3390/molecules26113417
New Oxaliplatin-Pyrophosphato Analogs with Improved In Vitro Cytotoxicity
Abstract
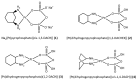
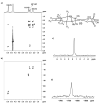
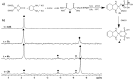
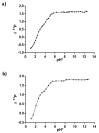
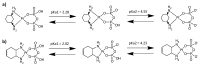
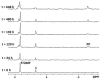
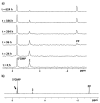
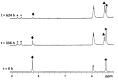
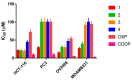
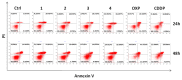
Two new Pt(II)-pyrophosphato complexes containing the carrier ligands cis-1,3-diaminocyclohexane (cis-1,3-DACH) and trans-1,2-diamine-4-cyclohexene (1,2-DACHEX), variants of the 1R,2R-diaminocyclohexane ligand present in the clinically used Pt-drug oxaliplatin, have been synthesized with the aim of developing new potential antitumor drugs with high bone tropism. The complexes are more stable at physiological pH than in acid conditions, with Na2[Pt(pyrophosphato)(cis-1,3-DACH)] (1) slightly more stable than [Pt(dihydrogenpyrophosphato)(1,2-DACHEX)] (2). The greater reactivity at acidic pH ensures a greater efficacy at the tumor site. Preliminary NMR studies indicate that 1 and 2 react slowly with 5'-GMP (used as a model of nucleic acids), releasing the pyrophosphate ligand and affording the bis 5'-GMP adduct. In vitro cytotoxicity assays performed against a panel of four human cancer cell lines have shown that both compounds are more active than oxaliplatin. Flow cytometry studies on HCT116 cells showed that the pyrophosphato compounds with the non-classical 1,3- and 1,4-diaminocyclohexane ligands (1 and 4) are the most capable to induce cells' death by apoptosis and necrosis.
Keywords: antitumor drugs; bone tumors; cisplatin; oxaliplatin; phosphaplatins; pyrophosphate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
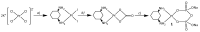



References
-
- Anthony E.J., Bolitho E.M., Bridgewater H.E., Carter O.W.L., Donnelly J.M., Imberti C., Lant E.C., Lermyte F., Needham R.J., Palau M., et al. Metallodrugs are unique: Opportunities and challenges of discovery and development. Chem. Sci. 2020;11:12888–12917. doi: 10.1039/D0SC04082G. - DOI - PMC - PubMed
-
- Uchino H., Matsumura Y., Negishi T., Koizumi F., Hayashi T., Honda T., Nishiyama N., Kataoka K., Naito S., Kakizoe T. Cisplatin-incorporating polymeric micelles (NC-6004) can reduce nephrotoxicity and neurotoxicity of cisplatin in rats. Br. J. Cancer. 2005;93:678–687. doi: 10.1038/sj.bjc.6602772. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

