Informal Caregiving in Amyotrophic Lateral Sclerosis (ALS): A High Caregiver Burden and Drastic Consequences on Caregivers' Lives
- PMID: 34200087
- PMCID: PMC8228206
- DOI: 10.3390/brainsci11060748
Informal Caregiving in Amyotrophic Lateral Sclerosis (ALS): A High Caregiver Burden and Drastic Consequences on Caregivers' Lives
Abstract
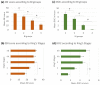
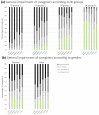
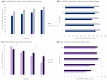
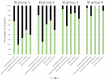
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease that causes progressive autonomy loss and need for care. This does not only affect patients themselves, but also the patients' informal caregivers (CGs) in their health, personal and professional lives. The big efforts of this multi-center study were not only to evaluate the caregivers' burden and to identify its predictors, but it also should provide a specific understanding of the needs of ALS patients' CGs and fill the gap of knowledge on their personal and work lives. Using standardized questionnaires, primary data from patients and their main informal CGs (n = 249) were collected. Patients' functional status and disease severity were evaluated using the Barthel Index, the revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) and the King's Stages for ALS. The caregivers' burden was recorded by the Zarit Burden Interview (ZBI). Comorbid anxiety and depression of caregivers were assessed by the Hospital Anxiety and Depression Scale. Additionally, the EuroQol Five Dimension Five Level Scale evaluated their health-related quality of life. The caregivers' burden was high (mean ZBI = 26/88, 0 = no burden, ≥24 = highly burdened) and correlated with patients' functional status (rp = -0.555, p < 0.001, n = 242). It was influenced by the CGs' own mental health issues due to caregiving (+11.36, 95% CI [6.84; 15.87], p < 0.001), patients' wheelchair dependency (+9.30, 95% CI [5.94; 12.66], p < 0.001) and was interrelated with the CGs' depression (rp = 0.627, p < 0.001, n = 234), anxiety (rp = 0.550, p < 0.001, n = 234), and poorer physical condition (rp = -0.362, p < 0.001, n = 237). Moreover, female CGs showed symptoms of anxiety more often, which also correlated with the patients' impairment in daily routine (rs = -0.280, p < 0.001, n = 169). As increasing disease severity, along with decreasing autonomy, was the main predictor of caregiver burden and showed to create relevant (negative) implications on CGs' lives, patient care and supportive therapies should address this issue. Moreover, in order to preserve the mental and physical health of the CGs, new concepts of care have to focus on both, on not only patients but also their CGs and gender-associated specific issues. As caregiving in ALS also significantly influences the socioeconomic status by restrictions in CGs' work lives and income, and the main reported needs being lack of psychological support and a high bureaucracy, the situation of CGs needs more attention. Apart from their own multi-disciplinary medical and psychological care, more support in care and patient management issues is required.
Keywords: amyotrophic lateral sclerosis (ALS); anxiety; caregiver burden; decreasing autonomy; depression; functional status; health-related quality of life; informal caregiving; psychological support; socioeconomic status.
Conflict of interest statement
P.S. reports no conflicts of interest. I.C. reports no conflicts of interest. R.G. reports no conflicts of interest. B.S. received speaker honoraria from Biogen without relation to ALS. D.Z. received compensation for participation on an advisory board of Biogen, as well as for consultancy work from Novartis. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. C.S. (Carsten Schröter) reports no conflicts of interest. U.W. received compensation for activities with Merz and Desitin. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. M.R. reports no conflicts of interest. J.W. reports no conflicts of interest. I.S. received personal/speaker fees from Sanofi Genzyme, Biogen and from the German Neuromuscular Society “Deutsche Gesellschaft fuer Muskelkranke“ (DGM e.V.) without relation to ALS. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. A.H. reported conflict of interest due to consultant work for Biogen, Desitin, and Roche. Additionally, A.H. received research funding from BMBF, GBA-Innovations Fund, Helmholtz Gesellschaft, NOMIS-Foundation, and “Hermann und Lilly-Schilling Stiftung für medizinische Forschung” im Stifterverband. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. M.M. reports no conflicts of interest. Z.K. reports no conflicts of interest. R.A.L. received compensation for activities with Biogen, Celgene, Merck, Novartis, and Roche, as well as research support from DFG, Else-Kröner-Fresenius Stiftung, Biogen and Novartis. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. J.C.K. received personal fees from Biogen, Roche, Avexis, Abbvie and Ipsen and funding by the “Deutsche Gesellschaft fuer Muskelkranke “(DGM e.V.) outside of the submitted work. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. C.S. (Claudia Stendel) reports no conflicts of interest. L.H.M. reports no conflicts of interest. A.O. received honoraria as a speaker/consultant from the German Neuromuscular Society “Deutsche Gesellschaft fuer Muskelkranke “(DGM e.V.), Biogen and Impulze. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. C.B. reports no conflicts of interest. T.K. reports no conflicts of interest. J.D. reports no conflicts of interest. A.C.L. reports no conflicts of interest. M.B. received honoraria from UCB Pharma, Sanofi Genzyme, Desitin, Löwenstein Medical, Sanofi- Genzyme, and Biogen, as well as financial research support from Sanofi-Genzyme and Löwenstein Medical. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. T.H. received honoraria from Biogen, Roche, Novartis and Alexion without relation to ALS and received research funding from Biogen, AveXis and Roche without relation to ALS. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. M.D. received honoraria from Biogen and Roche without relation to ALS and received funding for the BMBF-funded Rock ALS study and for the registered study SMArtCARE from the University of Freiburg (without relation to ALS). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. P.L. has no conflicts of interest in the context of this work. S.P. received honoraria as speaker/consultant from Biogen GmbH, Roche, Novartis, Teva, Cytokinetics Inc., and Desitin; and grants from DGM e.V, Federal Ministry of Education and Research, German Israeli Foundation for Scientific Research and Development, EU Joint Program for Neurodegenerative Disease Research. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. O.S.-K. has received honoraria as a speaker/consultant and/or funding for travel expenses from the German Neuromuscular Society “Deutsche Gesellschaft fuer Muskelkranke (DGM e.V.), Novartis, Biogen GmbH, Biermann Verlag GmbH, MK+S—Medizin, Kommunikation & Service GmbH, and the Jain Foundation. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Abe K., Itoyama Y., Sobue G., Tsuji S., Aoki M., Doyu M., Hamada C., Kondo K., Yoneoka T., Akimoto M., et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph. Lateral Scler. Front. Degener. 2014;15:610–617. doi: 10.3109/21678421.2014.959024. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

