Fabrication of Capsaicin Loaded Nanocrystals: Physical Characterizations and In Vivo Evaluation
- PMID: 34200268
- PMCID: PMC8227085
- DOI: 10.3390/pharmaceutics13060841
Fabrication of Capsaicin Loaded Nanocrystals: Physical Characterizations and In Vivo Evaluation
Abstract
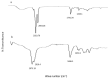
Nano-crystallization is a new emerging strategy to promote the saturation solubility, dissolution rate and subsequent bioavailability of Biopharmaceutical Class II drugs. Capsaicin belongs to BCS class-II drugs having low water solubility and dissolution rate. Nano-crystals (NC) of pure Capsaicin was developed and optimized in order to increase its water solubility, dissolution and further to promote its adhesiveness to skin epidermis layer. NC formulations were subjected to stability studies, droplet size, surface charge, poly-dispensability index, drug content, entrapment efficiency, thermal analysis, surface morphology, crystalline studies, solubility profile, in vitro release and ex vivo permeation studies. In vivo anti-inflammatory assay (Carrageenan-induced paw edema) was performed in Sprague Dawley rats. Nanocrystals loaded with capsaicin showed particle size 120 ± 3.0 nm with surface charge of -20.7 ± 3.5 and PDI was 0.48 ± 1.5. Drug content and entrapment efficiency of T3 was 85% and 90 ± 1.9% respectively. Thermal studies predicted that melting peak of capsaicin was present in the formulation suggested that there was no interaction between active moieties and excipients in NC formulation. Surface morphology confirmed the presence of Nano-size crystals having rough crystalline surface. XRD proved that the capsaicin NC are successfully developed by using high speed homogenization. The solubility of capsaicin was found to be 12.0 ± 0.013 μg/mL in water. In vitro study revealed that 89.94 ± 1.9% of drug was released within 24 h. Similarly, drug permeation was 68.32 ± 1.83%, drug retained in skin was 16.13 ± 1.11% while drug retained on skin was 9.12 ± 0.14% after 12 h. The nanocrystals showed higher anti-inflammatory activity as compared to marketed product (Dicloran®). The study concluded that improvement in dissolution rate of capsaicin may potentially provide the opportunities in the development of a much cost-effective dosage forms that will produce improved pharmacological effects, but at low dose as compared to the already available products.
Keywords: BCS class II drugs; capsaicin; nanocrystals; solubility enhancement; top down technique.
Conflict of interest statement
All the authors declare that they have no competing interest.
Figures







References
-
- Wu X., Guy R.H. Applications of nanoparticles in topical drug delivery and in cosmetics. J. Drug Deliv. Sci. Technol. 2009;19:371–384. doi: 10.1016/S1773-2247(09)50080-9. - DOI
-
- Shete G., Jain H., Punj D., Prajapat H., Akotiya P., Bansal A.K. Stabilizers used in nanocrystal based drug delivery systems. J. Excip. Food Chem. 2014;5:184–209.
-
- Müller R.H., Zhai X., Romero G.B., Keck C.M. Nanocrystals for passive dermal penetration enhancement. In: Dragicevic N., Maibach H.I., editors. Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Nanocarriers. 1st ed. Springer; Berlin/Heidelberg, Germany: 2016. pp. 283–295.
LinkOut - more resources
Full Text Sources
Other Literature Sources

