A New Ultrasensitive Bioluminescence-Based Method for Assaying Monoacylglycerol Lipase
- PMID: 34200322
- PMCID: PMC8200976
- DOI: 10.3390/ijms22116148
A New Ultrasensitive Bioluminescence-Based Method for Assaying Monoacylglycerol Lipase
Abstract
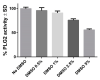
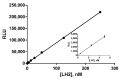
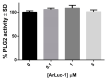
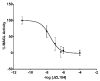
A novel bioluminescent Monoacylglycerol lipase (MAGL) substrate 6-O-arachidonoylluciferin, a D-luciferin derivative, was synthesized, physico-chemically characterized, and used as highly sensitive substrate for MAGL in an assay developed for this purpose. We present here a new method based on the enzymatic cleavage of arachidonic acid with luciferin release using human Monoacylglycerol lipase (hMAGL) followed by its reaction with a chimeric luciferase, PLG2, to produce bioluminescence. Enzymatic cleavage of the new substrate by MAGL was demonstrated, and kinetic constants Km and Vmax were determined. 6-O-arachidonoylluciferin has proved to be a highly sensitive substrate for MAGL. The bioluminescence assay (LOD 90 pM, LOQ 300 pM) is much more sensitive and should suffer fewer biological interferences in cells lysate applications than typical fluorometric methods. The assay was validated for the identification and characterization of MAGL modulators using the well-known MAGL inhibitor JZL184. The use of PLG2 displaying distinct bioluminescence color and kinetics may offer a highly desirable opportunity to extend the range of applications to cell-based assays.
Keywords: PLG2; bioluminescence; kinetic assay; monoacylglycerol lipase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Ottria R., Cappelletti L., Ravelli A., Mariotti M., Gigli F., Romagnoli S., Ciuffreda P., Banfi G., Drago L. Plasma endocannabinoid behaviour in total knee and hip arthroplasty. J. Biol. Regul. Homeost. Agents. 2016;30:1147–1152. - PubMed
-
- Pezzilli R., Ciuffreda P., Ottria R., Ravelli A., Melzi d’Eril G., Barassi A. Serum endocannabinoids in assessing pain in patients with chronic pancreatitis and in those with pancreatic ductal adenocarcinoma. Scand. J. Gastroenterol. 2017;52:1133–1139. doi: 10.1080/00365521.2017.1342139. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

