Exosome-Derived MicroRNAs of Human Milk and Their Effects on Infant Health and Development
- PMID: 34200323
- PMCID: PMC8228670
- DOI: 10.3390/biom11060851
Exosome-Derived MicroRNAs of Human Milk and Their Effects on Infant Health and Development
Abstract
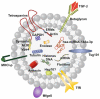
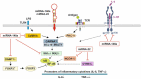
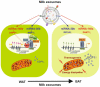
Multiple biologically active components of human milk support infant growth, health and development. Milk provides a wide spectrum of mammary epithelial cell-derived extracellular vesicles (MEVs) for the infant. Although the whole spectrum of MEVs appears to be of functional importance for the growing infant, the majority of recent studies report on the MEV subfraction of milk exosomes (MEX) and their miRNA cargo, which are in the focus of this review. MEX and the dominant miRNA-148a play a key role in intestinal maturation, barrier function and suppression of nuclear factor-κB (NF-κB) signaling and may thus be helpful for the prevention and treatment of necrotizing enterocolitis. MEX and their miRNAs reach the systemic circulation and may impact epigenetic programming of various organs including the liver, thymus, brain, pancreatic islets, beige, brown and white adipose tissue as well as bones. Translational evidence indicates that MEX and their miRNAs control the expression of global cellular regulators such as DNA methyltransferase 1-which is important for the up-regulation of developmental genes including insulin, insulin-like growth factor-1, α-synuclein and forkhead box P3-and receptor-interacting protein 140, which is important for the regulation of multiple nuclear receptors. MEX-derived miRNA-148a and miRNA-30b may stimulate the expression of uncoupling protein 1, the key inducer of thermogenesis converting white into beige/brown adipose tissue. MEX have to be considered as signalosomes derived from the maternal lactation genome emitted to promote growth, maturation, immunological and metabolic programming of the offspring. Deeper insights into milk's molecular biology allow the conclusion that infants are both "breast-fed" and "breast-programmed". In this regard, MEX miRNA-deficient artificial formula is not an adequate substitute for breastfeeding, the birthright of all mammals.
Keywords: DNA methyltransferase 1; adipogenesis; immune tolerance; intestinal maturation; milk exosome; milk miRNAs; necrotizing enterocolitis; nuclear factor-κB; receptor-interacting protein 140; systemic milk effects.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
MicroRNAs: Milk's epigenetic regulators.Best Pract Res Clin Endocrinol Metab. 2017 Aug;31(4):427-442. doi: 10.1016/j.beem.2017.10.003. Epub 2017 Oct 20. Best Pract Res Clin Endocrinol Metab. 2017. PMID: 29221571 Review.
-
Regulation of adipogenesis by exosomal milk miRNA.Rev Endocr Metab Disord. 2023 Apr;24(2):297-316. doi: 10.1007/s11154-023-09788-3. Epub 2023 Jan 24. Rev Endocr Metab Disord. 2023. PMID: 36692804 Review.
-
Milk Exosomal microRNAs: Postnatal Promoters of β Cell Proliferation but Potential Inducers of β Cell De-Differentiation in Adult Life.Int J Mol Sci. 2022 Sep 29;23(19):11503. doi: 10.3390/ijms231911503. Int J Mol Sci. 2022. PMID: 36232796 Free PMC article. Review.
-
Porcine Milk Exosome MiRNAs Attenuate LPS-Induced Apoptosis through Inhibiting TLR4/NF-κB and p53 Pathways in Intestinal Epithelial Cells.J Agric Food Chem. 2019 Aug 28;67(34):9477-9491. doi: 10.1021/acs.jafc.9b02925. Epub 2019 Aug 20. J Agric Food Chem. 2019. PMID: 31429552
-
Synergistic Effects of Milk-Derived Exosomes and Galactose on α-Synuclein Pathology in Parkinson's Disease and Type 2 Diabetes Mellitus.Int J Mol Sci. 2021 Jan 21;22(3):1059. doi: 10.3390/ijms22031059. Int J Mol Sci. 2021. PMID: 33494388 Free PMC article. Review.
Cited by
-
Pig Milk Exosome Packaging ssc-miR-22-3p Alleviates Pig Intestinal Epithelial Cell Injury and Inflammatory Response by Targeting MAPK14.Int J Mol Sci. 2024 Oct 5;25(19):10715. doi: 10.3390/ijms251910715. Int J Mol Sci. 2024. PMID: 39409044 Free PMC article.
-
Identification of omega-3 oxylipins in human milk-derived extracellular vesicles with pro-resolutive actions in gastrointestinal inflammation.Front Immunol. 2023 Nov 20;14:1293737. doi: 10.3389/fimmu.2023.1293737. eCollection 2023. Front Immunol. 2023. PMID: 38054009 Free PMC article.
-
Breast milk microRNAs: Potential players in oral tolerance development.Front Immunol. 2023 Mar 14;14:1154211. doi: 10.3389/fimmu.2023.1154211. eCollection 2023. Front Immunol. 2023. PMID: 36999032 Free PMC article. Review.
-
Prevention of Chronic Morbidities in Extremely Premature Newborns with LISA-nCPAP Respiratory Therapy and Adjuvant Perinatal Strategies.Antioxidants (Basel). 2023 May 24;12(6):1149. doi: 10.3390/antiox12061149. Antioxidants (Basel). 2023. PMID: 37371878 Free PMC article. Review.
-
The association of human milk intake and outcomes in biliary atresia.J Pediatr Gastroenterol Nutr. 2025 Jan;80(1):163-173. doi: 10.1002/jpn3.12403. Epub 2024 Nov 11. J Pediatr Gastroenterol Nutr. 2025. PMID: 39526563
References
-
- Zonneveld M.I., van Herwijnen M.J.C., Fernandez-Gutierrez M.M., Giovanazzi A., de Groot A.M., Kleinjan M., van Capel T.M.M., Sijts A.J.A.M., Taams L.S., Garssen J., et al. Human milk extracellular vesicles target nodes in interconnected signalling pathways that enhance oral epithelial barrier function and dampen immune responses. J. Extracell. Vesicles. 2021;10:e12071. doi: 10.1002/jev2.12071. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

