Nucleic Acid Testing of SARS-CoV-2
- PMID: 34200331
- PMCID: PMC8201071
- DOI: 10.3390/ijms22116150
Nucleic Acid Testing of SARS-CoV-2
Abstract
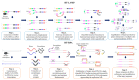
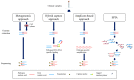
The coronavirus disease 2019 (COVID-19) has caused a large global outbreak. It is accordingly important to develop accurate and rapid diagnostic methods. The polymerase chain reaction (PCR)-based method including reverse transcription-polymerase chain reaction (RT-PCR) is the most widely used assay for the detection of SARS-CoV-2 RNA. Along with the RT-PCR method, digital PCR has emerged as a powerful tool to quantify nucleic acid of the virus with high accuracy and sensitivity. Non-PCR based techniques such as reverse transcription loop-mediated isothermal amplification (RT-LAMP) and reverse transcription recombinase polymerase amplification (RT-RPA) are considered to be rapid and simple nucleic acid detection methods and were reviewed in this paper. Non-conventional molecular diagnostic methods including next-generation sequencing (NGS), CRISPR-based assays and nanotechnology are improving the accuracy and sensitivity of COVID-19 diagnosis. In this review, we also focus on standardization of SARS-CoV-2 nucleic acid testing and the activity of the National Metrology Institutes (NMIs) and highlight resources such as reference materials (RM) that provide the values of specified properties. Finally, we summarize the useful resources for convenient COVID-19 molecular diagnostics.
Keywords: PCR; SARS-CoV-2; genome sequencing; isothermal amplification; nucleic acid testing; reference materials.
Conflict of interest statement
Seil Kim and Hee-Min Yoo are producers of KRISS SARS-CoV-2 reference materials.
Figures

References
-
- Vijgen L., Keyaerts E., Moës E., Thoelen I., Wollants E., Lemey P., Vandamme A.-M., Van Ranst M. Complete Genomic Sequence of Human Coronavirus OC43: Molecular Clock Analysis Suggests a Relatively Recent Zoonotic Coronavirus Transmission Event. J. Virol. 2005;79:1595–1604. doi: 10.1128/JVI.79.3.1595-1604.2005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

