Characterisation of Aspergillus fumigatus Endocytic Trafficking within Airway Epithelial Cells Using High-Resolution Automated Quantitative Confocal Microscopy
- PMID: 34200399
- PMCID: PMC8229978
- DOI: 10.3390/jof7060454
Characterisation of Aspergillus fumigatus Endocytic Trafficking within Airway Epithelial Cells Using High-Resolution Automated Quantitative Confocal Microscopy
Abstract
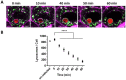
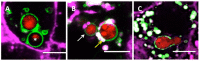
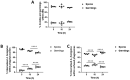
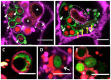
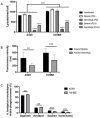
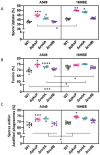
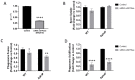
The precise characterization of the mechanisms modulating Aspergillus fumigatus survival within airway epithelial cells has been impaired by the lack of live-cell imaging technologies and user-friendly quantification approaches. Here we described the use of an automated image analysis pipeline to estimate the proportion of A. fumigatus spores taken up by airway epithelial cells, those contained within phagolysosomes or acidified phagosomes, along with the fungal factors contributing to these processes. Coupling the use of fluorescent A. fumigatus strains and fluorescent epithelial probes targeting lysosomes, acidified compartments and cell membrane, we found that both the efficacy of lysosome recruitment to phagosomes and phagosome acidification determines the capacity of airway epithelial cells to contain A. fumigatus growth. Overall, the capability of the airway epithelium to prevent A. fumigatus survival was higher in bronchial epithelial than alveolar epithelial cells. Certain A. fumigatus cell wall mutants influenced phagosome maturation in airway epithelial cells. Taken together, this live-cell 4D imaging approach allows observation and measurement of the very early processes of A. fumigatus interaction within live airway epithelial monolayers.
Keywords: Aspergillus fumigatus; airway epithelial cells; phagocytosis.
Conflict of interest statement
In the past five years, S.G. has received research funds from Pfizer and has been a council member of the International Society of Human and Animal Mycology (ISHAM). Denning and family hold Founder shares in F2G Ltd, a University of Manchester spin-out antifungal discovery company. He acts, or has recently acted, as a consultant to Pulmatrix, Pulmocide, Zambon, iCo Therapeutics, Mayne Pharma, Biosergen, Bright Angel Therapeutics, Cipla and Metis. He sits on the DSMB for a SARS CoV2 vaccine trial. In the last three years, he has been paid for talks on behalf of Dynamiker, Hikma, Gilead, Merck, Mylan and Pfizer. In the last 5 years D.T has received research funds from Gilead Science and has acted as a consultant for OwlStone Medical.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

