Mesenchymal Stem Cells as a Gene Delivery Tool: Promise, Problems, and Prospects
- PMID: 34200425
- PMCID: PMC8229096
- DOI: 10.3390/pharmaceutics13060843
Mesenchymal Stem Cells as a Gene Delivery Tool: Promise, Problems, and Prospects
Abstract
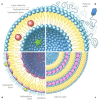
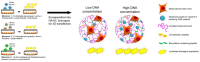
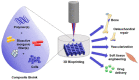
The cell-based approach in gene therapy arises as a promising strategy to provide safe, targeted, and efficient gene delivery. Owing to their unique features, as homing and tumor-tropism, mesenchymal stem cells (MSCs) have recently been introduced as an encouraging vehicle in gene therapy. Nevertheless, non-viral transfer of nucleic acids into MSCs remains limited due to various factors related to the main stakeholders of the process (e.g., nucleic acids, carriers, or cells). In this review, we have summarized the main types of nucleic acids used to transfect MSCs, the pros and cons, and applications of each. Then, we have emphasized on the most efficient lipid-based carriers for nucleic acids to MSCs, their main features, and some of their applications. While a myriad of studies have demonstrated the therapeutic potential for engineered MSCs therapy in various illnesses, optimization for clinical use is an ongoing challenge. On the way of improvement, genetically modified MSCs have been combined with various novel techniques and tools (e.g., exosomes, spheroids, 3D-Bioprinting, etc.,) aiming for more efficient and safe applications in biomedicine.
Keywords: 3D-bioprinting; COVID-19; cell therapy; gene therapy; mesenchymal stem cell; niosome; non-viral gene delivery; transfection.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
-
- Attia N., Mashal M. Mesenchymal Stem Cells: The Past Present and Future. Spinger; New York, NY, USA: 2020. - PubMed
-
- Mehanna R.A., Nabil I., Attia N., Bary A.A., Razek K.A., Ahmed T.A., Elsayed F. The effect of bone marrow-derived mesenchymal stem cells and their conditioned media topically delivered in fibrin glue on chronic wound healing in rats. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/846062. - DOI - PMC - PubMed
-
- Afanasyev B.V., Elstner E.E., Zander A.R. AJ Friedenstein, founder of the mesenchymal stem cell concept. Cell Ther. Transpl. 2009;1:35–38.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

